Small study effects in meta-analyses of osteoarthritis trials: meta-epidemiological study
- PMID: 20639294
- PMCID: PMC2905513
- DOI: 10.1136/bmj.c3515
Small study effects in meta-analyses of osteoarthritis trials: meta-epidemiological study
Abstract
Objective: To examine the presence and extent of small study effects in clinical osteoarthritis research.
Design: Meta-epidemiological study.
Data sources: 13 meta-analyses including 153 randomised trials (41 605 patients) that compared therapeutic interventions with placebo or non-intervention control in patients with osteoarthritis of the hip or knee and used patients' reported pain as an outcome.
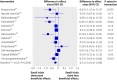
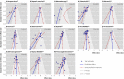
Methods: We compared estimated benefits of treatment between large trials (at least 100 patients per arm) and small trials, explored funnel plots supplemented with lines of predicted effects and contours of significance, and used three approaches to estimate treatment effects: meta-analyses including all trials irrespective of sample size, meta-analyses restricted to large trials, and treatment effects predicted for large trials.
Results: On average, treatment effects were more beneficial in small than in large trials (difference in effect sizes -0.21, 95% confidence interval -0.34 to -0.08, P=0.001). Depending on criteria used, six to eight funnel plots indicated small study effects. In six of 13 meta-analyses, the overall pooled estimate suggested a clinically relevant, significant benefit of treatment, whereas analyses restricted to large trials and predicted effects in large trials yielded smaller non-significant estimates.
Conclusions: Small study effects can often distort results of meta-analyses. The influence of small trials on estimated treatment effects should be routinely assessed.
Conflict of interest statement
Competing interests: All authors have completed the Unified Competing Interest form at
Figures

Comment in
-
Small studies in meta-analyses. Making the best of a little.BMJ. 2010 Aug 24;341:c4463. doi: 10.1136/bmj.c4463. BMJ. 2010. PMID: 20736275 No abstract available.
References
-
- Sterne JA, Gavaghan D, Egger M. Publication and related bias in meta-analysis: power of statistical tests and prevalence in the literature. J Clin Epidemiol 2000;53:1119-29. - PubMed
-
- Chan AW, Hrobjartsson A, Haahr MT, Gotzsche PC, Altman DG. Empirical evidence for selective reporting of outcomes in randomized trials: comparison of protocols to published articles. JAMA 2004;291:2457-65. - PubMed
