Genetic polymorphisms in organic cation transporter 1 (OCT1) in Chinese and Japanese populations exhibit altered function
- PMID: 20639304
- PMCID: PMC2957788
- DOI: 10.1124/jpet.110.170159
Genetic polymorphisms in organic cation transporter 1 (OCT1) in Chinese and Japanese populations exhibit altered function
Abstract
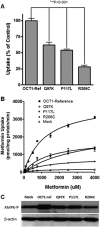
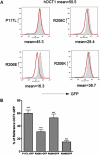
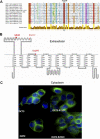
Organic cation transporter 1 (OCT1; SLC22A1) seems to play a role in the efficacy and disposition of the widely used antidiabetic drug metformin. Genetic variants in OCT1 have been identified largely in European populations. Metformin is increasingly being used in Asian populations where the incidence of type 2 diabetes (T2D) is on the rise. The goal of this study is to identify genetic variants of OCT1 in Chinese and Japanese populations, which may potentially modulate response to metformin. We used recent data from the 1000 Genomes Project (Chinese and Japanese) and direct sequencing of selected amplicons of OCT1 in 66 DNA samples from Japanese patients with T2D. A total of six nonsynonymous variants were identified. Three of them (Q97K, P117L, and R206C) had not been functionally characterized previously and had allele frequencies of 0.017, 0.023 and 0.008, respectively. The uptake of metformin in cells expressing Q97K, P117L, and R206C was significantly reduced relative to the OCT1 reference (62 ± 4.3, 55 ± 6.8, and 22 ± 1.5% for Q97K, P117L, and R206C, respectively). Kinetic studies indicated that P117L and R206C exhibited a reduced V(max), whereas Q97K showed an increased K(m). The green fluorescent protein (GFP)-tagged Q97K and P117L variants localized to the plasma membrane, whereas the GFP-tagged R206C was retained mainly in the endoplasmic reticulum. Replacement of the highly conserved R206 with different amino acids modulated the subcellular localization and function of the transporter. This study suggests that nonsynonymous variants of OCT1 in Chinese and Japanese populations may affect the differential response to metformin.
Figures


References
-
- Becker ML, Visser LE, van Schaik RH, Hofman A, Uitterlinden AG, Stricker BH. (2009) Genetic variation in the organic cation transporter 1 is associated with metformin response in patients with diabetes mellitus. Pharmacogenomics J 9:242–247 - PubMed
-
- Chen L, Durkin KA, Casida JE. (2006) Spontaneous mobility of GABAA receptor M2 extracellular half relative to noncompetitive antagonist action. J Biol Chem 281:38871–38878 - PubMed
-
- Itoda M, Saito Y, Maekawa K, Hichiya H, Komamura K, Kamakura S, Kitakaze M, Tomoike H, Ueno K, Ozawa S, et al. (2004) Seven novel single nucleotide polymorphisms in the human SLC22A1 gene encoding organic cation transporter 1 (OCT1). Drug Metab Pharmacokinet 19:308–312 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

