Aromatic acid metabolites of Escherichia coli K-12 can induce the marRAB operon
- PMID: 20639340
- PMCID: PMC2937402
- DOI: 10.1128/JB.00371-10
Aromatic acid metabolites of Escherichia coli K-12 can induce the marRAB operon
Abstract
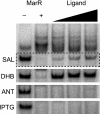
MarR is a key regulator of the marRAB operon involved in antibiotic resistance and solvent stress tolerance in Escherichia coli. We show that two metabolic intermediates, 2,3-dihydroxybenzoate and anthranilate, involved in enterobactin and tryptophan biosynthesis, respectively, can activate marRAB transcription. We also found that a third intermediate involved in ubiquinone biosynthesis, 4-hydroxybenzoate, activates marRAB transcription in the absence of TolC. Of the three, however, only 2,3-dihydroxybenzoate directly binds MarR and affects its activity.
Figures


References
-
- Alekshun, M. N., S. B. Levy, T. R. Mealy, B. A. Seaton, and J. F. Head. 2001. The crystal structure of MarR, a regulator of multiple antibiotic resistance, at 2.3 Å resolution. Nat. Struct. Biol. 8:710-714. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

