From the Cover: Charge interactions can dominate the dimensions of intrinsically disordered proteins
- PMID: 20639465
- PMCID: PMC2930438
- DOI: 10.1073/pnas.1001743107
From the Cover: Charge interactions can dominate the dimensions of intrinsically disordered proteins
Erratum in
- Proc Natl Acad Sci U S A. 2013 Oct 8;110(41):16693
Abstract
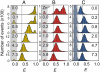
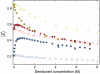
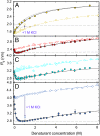
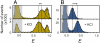
Many eukaryotic proteins are disordered under physiological conditions, and fold into ordered structures only on binding to their cellular targets. Such intrinsically disordered proteins (IDPs) often contain a large fraction of charged amino acids. Here, we use single-molecule Förster resonance energy transfer to investigate the influence of charged residues on the dimensions of unfolded and intrinsically disordered proteins. We find that, in contrast to the compact unfolded conformations that have been observed for many proteins at low denaturant concentration, IDPs can exhibit a prominent expansion at low ionic strength that correlates with their net charge. Charge-balanced polypeptides, however, can exhibit an additional collapse at low ionic strength, as predicted by polyampholyte theory from the attraction between opposite charges in the chain. The pronounced effect of charges on the dimensions of unfolded proteins has important implications for the cellular functions of IDPs.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Comment in
-
To fold or expand--a charged question.Proc Natl Acad Sci U S A. 2010 Aug 17;107(33):14519-20. doi: 10.1073/pnas.1008673107. Epub 2010 Aug 3. Proc Natl Acad Sci U S A. 2010. PMID: 20682745 Free PMC article. No abstract available.
References
-
- Dyson HJ, Wright PE. Intrinsically unstructured proteins and their functions. Nat Rev Mol Cell Biol. 2005;6:197–208. - PubMed
-
- Dunker AK, Silman I, Uversky VN, Sussman JL. Function and structure of inherently disordered proteins. Curr Opin Struct Biol. 2008;18:756–764. - PubMed
-
- Uversky VN, Oldfield CJ, Dunker AK. Intrinsically disordered proteins in human diseases: Introducing the D2 concept. Ann Rev Biophys. 2008;37:215–246. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

