In hepatic fibrosis, liver sinusoidal endothelial cells acquire enhanced immunogenicity
- PMID: 20639479
- PMCID: PMC3119346
- DOI: 10.4049/jimmunol.1000332
In hepatic fibrosis, liver sinusoidal endothelial cells acquire enhanced immunogenicity
Abstract
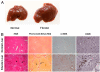
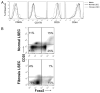
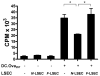
The normal liver is characterized by immunologic tolerance. Primary mediators of hepatic immune tolerance are liver sinusoidal endothelial cells (LSECs). LSECs block adaptive immunogenic responses to Ag and induce the generation of T regulatory cells. Hepatic fibrosis is characterized by both intense intrahepatic inflammation and altered hepatic immunity. We postulated that, in liver fibrosis, a reversal of LSEC function from tolerogenic to proinflammatory and immunogenic may contribute to both the heightened inflammatory milieu and altered intrahepatic immunity. We found that, after fibrotic liver injury from hepatotoxins, LSECs become highly proinflammatory and secrete an array of cytokines and chemokines. In addition, LSECs gain enhanced capacity to capture Ag and induce T cell proliferation. Similarly, unlike LSECs in normal livers, in fibrosis, LSECs do not veto dendritic cell priming of T cells. Furthermore, whereas in normal livers, LSECs are active in the generation of T regulatory cells, in hepatic fibrosis LSECs induce an immunogenic T cell phenotype capable of enhancing endogenous CTLs and generating potent de novo CTL responses. Moreover, depletion of LSECs from fibrotic liver cultures mitigates the proinflammatory milieu characteristic of hepatic fibrosis. Our findings offer a critical understanding of the role of LSECs in modulating intrahepatic immunity and inflammation in fibro-inflammatory liver disease.
Figures






References
-
- Elvevold K, Smedsrød B, Martinez I. The liver sinusoidal endothelial cell: a cell type of controversial and confusing identity. Am. J. Physiol. Gastrointest. Liver Physiol. 2008;294:G391–G400. - PubMed
-
- Katz SC, Pillarisetty VG, Bleier JI, Shah AB, DeMatteo RP. Liver sinusoidal endothelial cells are insufficient to activate T cells. J. Immunol. 2004;173:230–235. - PubMed
-
- Schurich A, Böttcher JP, Burgdorf S, Penzler P, Hegenbarth S, Kern M, Dolf A, Endl E, Schultze J, Wiertz E, et al. Distinct kinetics and dynamics of cross-presentation in liver sinusoidal endothelial cells compared to dendritic cells. Hepatology. 2009;50:909–919. - PubMed
-
- Knolle PA, Limmer A. Neighborhood politics: the immunoregulatory function of organ-resident liver endothelial cells. Trends Immunol. 2001;22:432–437. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

