Role of postnatal acquisition of the intestinal microbiome in the early development of immune function
- PMID: 20639773
- PMCID: PMC2932839
- DOI: 10.1097/MPG.0b013e3181e1a114
Role of postnatal acquisition of the intestinal microbiome in the early development of immune function
Abstract
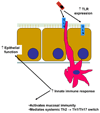
Objectives: Therapy with broad-spectrum antibiotics is a common practice for premature infants. This treatment can reduce the biodiversity of the fecal microbiota and may be a factor in the cause of necrotizing enterocolitis. In contrast, probiotic treatment of premature infants reduces the incidence of necrotizing enterocolitis. We hypothesized that 1 mechanism for these observations is the influence of bacteria on postnatal development of the mucosal immune system.
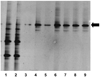
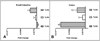
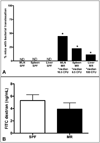
Materials and methods: Expression of immune molecules and microbial sensors was investigated in the postnatal mouse gastrointestinal tract by real-time polymerase chain reaction. Subsequently, 2-week-old specific pathogen-free and microbial-reduced (MR; antibiotic treated) mice were compared for immune molecule and microbial sensor expression, mesenteric lymph node T-cell numbers and activation, intestinal barrier function/permeability, systemic lymphocyte numbers, and T-cell phenotype commitment.
Results: Toll-like receptor 2, 4, and 5 expression was highest in 2-week-old specific pathogen-free mice, and this expression was decreased in MR mice. There was no difference in intestinal tight-junctional function, as evaluated by fluorescein isothiocyanate-dextran uptake, but MR mice had increased bacterial translocation across the intestinal epithelial barrier. MR mice had significantly fewer splenic B cells and mesenteric lymph node CD4+ T cells, but there were normal numbers of splenic T cells. These systemic T cells from MR mice produced more interleukin-4 and less interferon-gamma and IL-17, indicative of maintenance of the fetal, T-helper cell type 2 phenotype.
Conclusions: The present study shows that intestinal commensal microbiota have an influence on early postnatal immune development. Determining specific bacteria and/or bacterial ligands critical for this development could provide insight into the mechanisms by which broad-spectrum antibiotics and/or probiotic therapy influence the development of the mucosal immune system and mucosal-related diseases.
Figures





References
-
- Bäckhed F, Ley R, Sonnenburg J, Peterson D, Gordon J. Host-bacterial mutualism in the human intestine. Science. 2005;307(5717):1915–1920. - PubMed
-
- Hooper L, Midtvedt T, Gordon J. How host-microbial interactions shape the nutrient environment of the mammalian intestine. Annu Rev Nutr. 2002;22:283–307. - PubMed
-
- Levy O. Innate immunity of the newborn: basic mechanisms and clinical correlates. Nat Rev Immunol. 2007;7(5):379–390. - PubMed
-
- Trowsdale J, Betz A. Mother's little helpers: mechanisms of maternal-fetal tolerance. Nat Immunol. 2006;7(3):241–246. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

