Delivery of molecules into cells using carbon nanoparticles activated by femtosecond laser pulses
- PMID: 20639882
- PMCID: PMC2917490
- DOI: 10.1038/nnano.2010.126
Delivery of molecules into cells using carbon nanoparticles activated by femtosecond laser pulses
Abstract
A major barrier to drug and gene delivery is crossing the cell's plasma membrane. Physical forces applied to cells via electroporation, ultrasound and laser irradiation generate nanoscale holes in the plasma membrane for direct delivery of drugs into the cytoplasm. Inspired by previous work showing that laser excitation of carbon nanoparticles can drive the carbon-steam reaction to generate highly controlled shock waves, we show that carbon black nanoparticles activated by femtosecond laser pulses can facilitate the delivery of small molecules, proteins and DNA into two types of cells. Our initial results suggest that interaction between the laser energy and carbon black nanoparticles may generate photoacoustic forces by chemical reaction to create transient holes in the membrane for intracellular delivery.
Figures
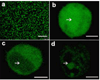
 ). MWNT were used to deliver calcein into DU145 cells (
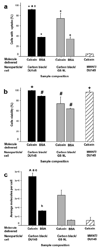
). MWNT were used to deliver calcein into DU145 cells ( ). Graphs show percentage of cells with intracellular uptake (a), cell viability (b), and average number of molecules delivered per cell (c), as a function of nanoparticle and cell type. CB and MWNT were added at final concentrations of 30µg/ml. Samples were irradiated at 5mJ/cm2 for 10 min. Data show average (n=3) ± SEM. *p<0.05 for cells with uptake of model drug compared to non-irradiated control (data not shown), #p<0.05 for cell viability compared to non-irradiated control, +p>0.05 for cell viability compared to non-irradiated control, ^p<0.05 for calcein molecule/cell compared to non-irradiated control, ap>0.05 for DU145 cells compared to GS-9L cells, bp<0.05 for DU145 cells compared to GS-9L, cp<0.05 for CB compared to MWNT. See S.I. Section 1.6 for statistical methods.
). Graphs show percentage of cells with intracellular uptake (a), cell viability (b), and average number of molecules delivered per cell (c), as a function of nanoparticle and cell type. CB and MWNT were added at final concentrations of 30µg/ml. Samples were irradiated at 5mJ/cm2 for 10 min. Data show average (n=3) ± SEM. *p<0.05 for cells with uptake of model drug compared to non-irradiated control (data not shown), #p<0.05 for cell viability compared to non-irradiated control, +p>0.05 for cell viability compared to non-irradiated control, ^p<0.05 for calcein molecule/cell compared to non-irradiated control, ap>0.05 for DU145 cells compared to GS-9L cells, bp<0.05 for DU145 cells compared to GS-9L, cp<0.05 for CB compared to MWNT. See S.I. Section 1.6 for statistical methods.
 ) of luciferase plasmid DNA in DU145 cells. Uptake of YOYO1-labeled plasmid DNA was assayed < 2h after irradiation to assess intracellular delivery of DNA molecules. Luciferase expression was measured 48h after irradiation to assess expression of the luciferase protein encoded in the DNA. Each sample had 5×105 cells and 30µg/ml CB. Irradiation was carried out at 5mJ/cm2 for 10 min. Non-irradiated controls were identical to irradiated samples, except no laser irradiation was applied. Data show average (n=3) ± SEM. *p<0.05 for treated cells compared to non-irradiated negative control.
) of luciferase plasmid DNA in DU145 cells. Uptake of YOYO1-labeled plasmid DNA was assayed < 2h after irradiation to assess intracellular delivery of DNA molecules. Luciferase expression was measured 48h after irradiation to assess expression of the luciferase protein encoded in the DNA. Each sample had 5×105 cells and 30µg/ml CB. Irradiation was carried out at 5mJ/cm2 for 10 min. Non-irradiated controls were identical to irradiated samples, except no laser irradiation was applied. Data show average (n=3) ± SEM. *p<0.05 for treated cells compared to non-irradiated negative control.

References
-
- Mitragotri S. Healing sound: the use of ultrasound in drug delivery and other therapeutic applications. Nat Rev Drug Discov. 2005;4:255–260. - PubMed
-
- Tirlapur UK, Konig K. Targeted transfection by femtosecond laser. Nature. 2002;418:290–291. - PubMed
-
- Kodama T, Doukas AG, Hamblin MR. Shock wave-mediated molecular delivery into cells. Biochim Biophys Acta. 2002;1542:186–194. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

