Still embedded together binding to membranes regulates Bcl-2 protein interactions
- PMID: 20639903
- PMCID: PMC6459407
- DOI: 10.1038/onc.2010.283
Still embedded together binding to membranes regulates Bcl-2 protein interactions
Abstract
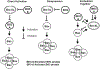
The dysregulation of apoptosis is a key step in developing tumours, and mediates resistance to cancer therapy. Many different signals for cell death converge on permeabilization of the outer mitochondrial membrane, which is controlled by the Bcl-2 family of proteins. The importance of this step is becoming increasingly relevant as the first generation of small molecules that inhibit the interaction of Bcl-2 family proteins enters clinical trials as anticancer agents. The Bcl-2 family can be divided into three classes: BH3-only proteins that are activated by various forms of cellular stress, Bax and Bak proteins that mediate mitochondrial membrane permeabilization, and inhibitory proteins such as Bcl-2 and Bcl-XL. The recently proposed embedded together model emphasizes the fact that many of the regulatory interactions between different classes of Bcl-2 family members occur at intracellular membranes, and binding to membranes causes conformational changes in the proteins that dictate functions in a dynamic manner. Within this context, recent results indicate that Bcl-XL functions as a dominant-negative Bax, a concept that resolves the paradox of similar structures but opposite functions of Bcl-XL and Bax. We have also shown that the conformational change that allows Bax to insert into the outer mitochondrial membrane is the rate-limiting step in the multistep process of Bax activation. Nevertheless, investigating the structure of activated Bax or Bak as monomers and as components of the oligomeric structures that mediate membrane permeabilization is the focus of ongoing research (and controversy) at many laboratories worldwide.
Conflict of interest statement
Conflict of interest
The authors declare no conflict of interest.
Figures
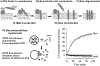
References
-
- Billen LP, Shamas-Din A, Andrews DW. (2009). Bid: a Bax-like BH3 protein. Oncogene 27: S93–S104. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

