An antiapoptotic neuroprotective role for neuroglobin
- PMID: 20640154
- PMCID: PMC2904918
- DOI: 10.3390/ijms11062306
An antiapoptotic neuroprotective role for neuroglobin
Abstract
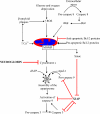
Cell death associated with mitochondrial dysfunction is common in acute neurological disorders and in neurodegenerative diseases. Neuronal apoptosis is regulated by multiple proteins, including neuroglobin, a small heme protein of ancient origin. Neuroglobin is found in high concentration in some neurons, and its high expression has been shown to promote survival of neurons in vitro and to protect brain from damage by both stroke and Alzheimer's disease in vivo. Early studies suggested this protective role might arise from the protein's capacity to bind oxygen or react with nitric oxide. Recent data, however, suggests that neither of these functions is likely to be of physiological significance. Other studies have shown that neuroglobin reacts very rapidly with cytochrome c released from mitochondria during cell death, thus interfering with the intrinsic pathway of apoptosis. Systems level computational modelling suggests that the physiological role of neuroglobin is to reset the trigger level for the post-mitochondrial execution of apoptosis. An understanding of the mechanism of action of neuroglobin might thus provide a rational basis for the design of new drug targets for inhibiting excessive neuronal cell death.
Keywords: apoptosis; cytochrome c; mitochondria; neuroglobin; systems biology.
Figures




References
-
- Galluzzi L, Blomgren K, Kroemer G. Mitochondrial membrane permeabilization in neuronal injury. Nat. Rev. Neurosci. 2009;10:481–494. - PubMed
-
- Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. - PubMed
-
- Skommer J, Wlodkowic D, Deptala A. Larger than life: Mitochondria and the Bcl-2 family. Leuk. Res. 2007;31:277–286. - PubMed
-
- Liston P, Fong WG, Korneluk RG. The inhibitors of apoptosis: There is more to life than Bcl2. Oncogene. 2003;22:8568–8580. - PubMed
-
- Lankester ER. A contribution to the knowledge of haemoglobion. Proc. Roy. Soc. 1872;21:70–81.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

