Advances towards synthetic machines at the molecular and nanoscale level
- PMID: 20640163
- PMCID: PMC2904927
- DOI: 10.3390/ijms11062453
Advances towards synthetic machines at the molecular and nanoscale level
Abstract
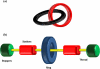
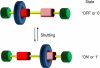
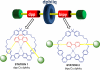
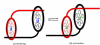
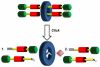
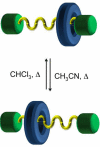
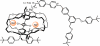
The fabrication of increasingly smaller machines to the nanometer scale can be achieved by either a "top-down" or "bottom-up" approach. While the former is reaching its limits of resolution, the latter is showing promise for the assembly of molecular components, in a comparable approach to natural systems, to produce functioning ensembles in a controlled and predetermined manner. In this review we focus on recent progress in molecular systems that act as molecular machine prototypes such as switches, motors, vehicles and logic operators.
Keywords: catenanes; devices; molecular machines; nanovehicles; rotary motors; rotaxanes; switches.
Figures

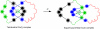







References
-
- Balzani V, Credi A, Raymo FM, Stoddart JF. Machines at the Molecular Level. Angew. Chem. Int. Ed. 2000;39:3348–3391. - PubMed
-
- Balzani V, Credi A, Stoddart JF. The Bottom-Up Approach to Molecular-Level Devices and Machines. Chem. Eur. J. 2002;8:5524–5532. - PubMed
-
- Ballardini R, Balzani V, Credi A, Gandolfi MT, Venturi M. Artificial Molecular-Level Machines: With Energy to Make them Work? Acc. Chem. Res. 2001;34:445–455. - PubMed
-
- Balzani V, Venturi M, Credi A. Molecular Devices and Machines. Wiley-VCH; Weinheim, Germany: 2003. pp. 269–277.
-
- Feynman RP. There’s Plenty of Room at the Bottom. Eng. Sci. 1960;23:22–36.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

