Nanocharacterization in dentistry
- PMID: 20640166
- PMCID: PMC2904930
- DOI: 10.3390/ijms11062523
Nanocharacterization in dentistry
Abstract
About 80% of US adults have some form of dental disease. There are a variety of new dental products available, ranging from implants to oral hygiene products that rely on nanoscale properties. Here, the application of AFM (Atomic Force Microscopy) and optical interferometry to a range of dentistry issues, including characterization of dental enamel, oral bacteria, biofilms and the role of surface proteins in biochemical and nanomechanical properties of bacterial adhesins, is reviewed. We also include studies of new products blocking dentine tubules to alleviate hypersensitivity; antimicrobial effects of mouthwash and characterizing nanoparticle coated dental implants. An outlook on future "nanodentistry" developments such as saliva exosomes based diagnostics, designing biocompatible, antimicrobial dental implants and personalized dental healthcare is presented.
Keywords: afm; bacterial adhesins; biofilms; dentine tubule; dentistry; implants; interferometry; nano-characterization; nanodentistry.
Figures

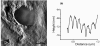

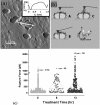
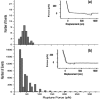
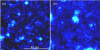



References
-
- Freitas RA., Jr Nanodentistry. J. Am. Dent. Assoc. 2000;131:1559–1565. - PubMed
-
- Dufrene YF. Towards nanomicrobiology using atomic force microscopy. Nat. Rev. Microbiol. 2008;6:674–680. - PubMed
-
- Kasas S, Fellay B, Cargnello R. Observation of the action of penicillin on bacillus-subtilis using atomic-force microscopy - technique for the preparation of bacteria. Surf. Interf. Anal. 1994;21:400–401.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

