Postnatal development of the endbulb of held in congenitally deaf cats
- PMID: 20640179
- PMCID: PMC2904654
- DOI: 10.3389/fnana.2010.00019
Postnatal development of the endbulb of held in congenitally deaf cats
Abstract
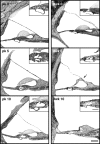
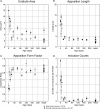
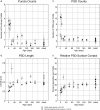
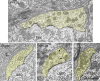
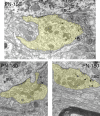
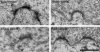
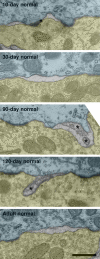
The endbulbs of Held are formed by the ascending branches of myelinated auditory nerve fibers and represent one of the largest synaptic endings in the brain. Normally, these endings are highly branched and each can form up to 1000 dome-shaped synapses. The deaf white cat is a model of congenital deafness involving a type of cochleosaccular degeneration that mimics the Scheibe deformity in humans. Endbulbs of mature deaf white cats exhibit reduced branching, hypertrophy of postsynaptic densities (PSDs), and changes in synaptic vesicle density. Because cats are essentially deaf at birth, we sought to determine if the progression of brain abnormalities was linked in time to the failure of normal hearing development. The rationale was that the lack of sound-evoked activity would trigger pathologic change in deaf kittens. The cochleae of deaf cats did not exhibit abnormal morphology at birth. After the first postnatal week, however, the presence of a collapsed scala media signaled the difference between deaf and hearing cats. By working backwards in age, endbulbs of deaf cats expressed flattened and elongated PSDs and increased synaptic vesicle density as compared to normal endbulbs. These differences are present at birth in some white kittens, presaging deafness despite their normal cochlear histology. We speculate that hearing pathology is signaled by a perinatal loss of spontaneous bursting activity in auditory nerve fibers or perhaps by some factor released by hair cell synapses before obliteration of the organ of Corti.
Keywords: auditory nerve; cochlear nucleus; hearing synapse; ultrastructure.
Figures







References
-
- Bosher S. K., Hallpike C. S. (1967). Observations on the histogenesis of the inner ear degeneration of the deaf white cat and its possible relationship to the aetiology of certain unexplained varieties of human congenital deafness. J. Laryngol. Otol. 80, 222–235 - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

