Immunoaffinity chromatography: an introduction to applications and recent developments
- PMID: 20640220
- PMCID: PMC2903764
- DOI: 10.4155/bio.10.31
Immunoaffinity chromatography: an introduction to applications and recent developments
Abstract
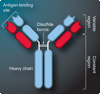
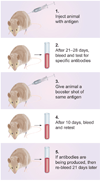
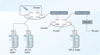
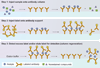
Immunoaffinity chromatography (IAC) combines the use of LC with the specific binding of antibodies or related agents. The resulting method can be used in assays for a particular target or for purification and concentration of analytes prior to further examination by another technique. This review discusses the history and principles of IAC and the various formats that can be used with this method. An overview is given of the general properties of antibodies and of antibody-production methods. The supports and immobilization methods used with antibodies in IAC and the selection of application and elution conditions for IAC are also discussed. Several applications of IAC are considered, including its use in purification, immunodepletion, direct sample analysis, chromatographic immunoassays and combined analysis methods. Recent developments include the use of IAC with CE or MS, ultrafast immunoextraction methods and the use of immunoaffinity columns in microanalytical systems.
Figures






References
-
-
Hage DS, Phillips TM. Chapter 6 Immunoaffinity chromatography. In: Hage DS, editor. Handbook of Affinity Chromatography. NY, USA: Taylor & Francis; 2006. . ▪ Provides an indepth look at immunoaffinity chromatography, with an emphasis on method development, immunoextraction and sample analysis by immunoaffinity chromatography.
-
-
-
Hage DS. Survey of recent advances in analytical applications of immunoaffinity chromatography. J. Chromatogr. B. 1998;715(1):3–28.. ▪ Provides a good overall review of immunoaffinity chromatography and its analytical applications.
-
-
- Calton GJ. Immunosorbent separations. Meth. Enzymol. 1985;104:381–387. - PubMed
-
- Phillips TM. High performance immunoaffinity chromatography. An introduction. LC Mag. 1985;3:962–972.
-
- D’allesandro G, Sofia F. The adsorption of antibodies from the sera of syphilitics and turburculosis patients. Z. Immunitats. 1935;84:237–250.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
