Cooperative NH⋯O and CH⋯O interactions for sulfate encapsulation in a thiophene-based macrocycle
- PMID: 20640221
- PMCID: PMC2903765
- DOI: 10.1016/j.tetlet.2010.06.036
Cooperative NH⋯O and CH⋯O interactions for sulfate encapsulation in a thiophene-based macrocycle
Abstract
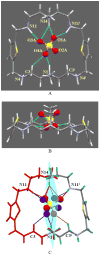
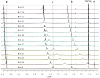
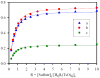
A thiophene-based macrocycle containing four secondary and two tertiary amines has been synthesized and its binding affinity has been investigated toward sulfate anion in solution and solid states. Structural analysis of the sulfate salt suggests that the ligand in its hexaprotonated form, is capable of encapsulating one sulfate within the cavity through cooperative NH⋯O and CH⋯O interactions. As investigated by (1)HNMR titrations, the lignad forms a 1:1 complex with sulfate in water at pH 2.1, showing a binding constant (K) of 3200 M(-1). The formation of the complex has been further confirmed by ESI-MS, indicating that the complex can exist in solution with a considerable stability.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
