Myo1c mutations associated with hearing loss cause defects in the interaction with nucleotide and actin
- PMID: 20640478
- PMCID: PMC3014424
- DOI: 10.1007/s00018-010-0448-x
Myo1c mutations associated with hearing loss cause defects in the interaction with nucleotide and actin
Abstract
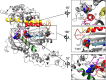
Three heterozygous missense mutations in the motor domain of myosin 1c (Myo1c), which mediates adaptation in the inner ear, are associated with bilateral sensorineural hearing loss in humans. With transient kinetic analyses, steady-state ATPase and motility assays, and homology modeling, we studied the interaction of these mutants with nucleotide and actin using a truncated construct, Myo1c(1IQ-SAH), which includes an artificial lever arm. Results indicate that mutation R156W, near switch 1, affects the nucleotide-binding pocket and the calcium binding by disrupting switch 1 movement. Mutation V252A, in the K helix of the upper 50 kDa domain, showed reduced actin affinity consistent with disruption of communication between the actin- and nucleotide-binding sites. T380M, in a Myo1c-specific insert in the HO linker, displayed aberrant changes in most kinetic parameters and uncoupling of the ATPase from motility. These data allow for an interpretation of how these mutations might affect adaptation.
Figures

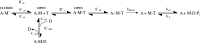


Similar articles
-
A hearing loss-associated myo1c mutation (R156W) decreases the myosin duty ratio and force sensitivity.Biochemistry. 2011 Mar 22;50(11):1831-8. doi: 10.1021/bi1016777. Epub 2011 Feb 15. Biochemistry. 2011. PMID: 21265502 Free PMC article.
-
Modification of loop 1 affects the nucleotide binding properties of Myo1c, the adaptation motor in the inner ear.Biochemistry. 2010 Feb 9;49(5):958-71. doi: 10.1021/bi901803j. Biochemistry. 2010. PMID: 20039646 Free PMC article.
-
Calcium, nucleotide, and actin affect the interaction of mammalian Myo1c with its light chain calmodulin.Biochemistry. 2008 Sep 23;47(38):10218-26. doi: 10.1021/bi8011059. Epub 2008 Aug 26. Biochemistry. 2008. PMID: 18729383
-
Drosophila class-I myosins that can impact left-right asymmetry have distinct ATPase kinetics.J Biol Chem. 2023 Aug;299(8):104961. doi: 10.1016/j.jbc.2023.104961. Epub 2023 Jun 26. J Biol Chem. 2023. PMID: 37380077 Free PMC article.
-
Mutating the converter-relay interface of Drosophila myosin perturbs ATPase activity, actin motility, myofibril stability and flight ability.J Mol Biol. 2010 May 21;398(5):625-32. doi: 10.1016/j.jmb.2010.03.049. Epub 2010 Apr 1. J Mol Biol. 2010. PMID: 20362584 Free PMC article.
Cited by
-
Loss of Motor Protein MYO1C Causes Rhodopsin Mislocalization and Results in Impaired Visual Function.Cells. 2021 May 26;10(6):1322. doi: 10.3390/cells10061322. Cells. 2021. PMID: 34073294 Free PMC article.
-
A hearing loss-associated myo1c mutation (R156W) decreases the myosin duty ratio and force sensitivity.Biochemistry. 2011 Mar 22;50(11):1831-8. doi: 10.1021/bi1016777. Epub 2011 Feb 15. Biochemistry. 2011. PMID: 21265502 Free PMC article.
-
Mechanochemical properties of human myosin-1C are modulated by isoform-specific differences in the N-terminal extension.J Biol Chem. 2021 Jan-Jun;296:100128. doi: 10.1074/jbc.RA120.015187. Epub 2020 Dec 3. J Biol Chem. 2021. PMID: 33257319 Free PMC article.
-
The duplication 17p13.3 phenotype: analysis of 21 families delineates developmental, behavioral and brain abnormalities, and rare variant phenotypes.Am J Med Genet A. 2013 Aug;161A(8):1833-52. doi: 10.1002/ajmg.a.35996. Epub 2013 Jun 27. Am J Med Genet A. 2013. PMID: 23813913 Free PMC article.
-
Functional Role of Class III Myosins in Hair Cells.Front Cell Dev Biol. 2021 Feb 25;9:643856. doi: 10.3389/fcell.2021.643856. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33718386 Free PMC article. Review.
References
-
- Hudspeth AJ. Hair-bundle mechanics and a model for mechanoelectrical transduction by hair cells. Soc Gen Physiol Ser. 1992;47:357–370. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

