B-cell activation influences T-cell polarization and outcome of anti-CD20 B-cell depletion in central nervous system autoimmunity
- PMID: 20641064
- PMCID: PMC3375897
- DOI: 10.1002/ana.22081
B-cell activation influences T-cell polarization and outcome of anti-CD20 B-cell depletion in central nervous system autoimmunity
Abstract
Objective: Clinical studies indicate that anti-CD20 B-cell depletion may be an effective multiple sclerosis (MS) therapy. We investigated mechanisms of anti-CD20-mediated immune modulation using 2 paradigms of experimental autoimmune encephalomyelitis (EAE).
Methods: Murine EAE was induced by recombinant myelin oligodendrocyte glycoprotein (rMOG), a model in which B cells are considered to contribute pathogenically, or MOG peptide (p)35-55, which does not require B cells.
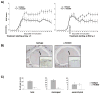
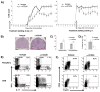
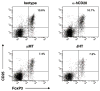
Results: In EAE induced by rMOG, B cells became activated and, when serving as antigen-presenting cells (APCs), promoted differentiation of proinflammatory MOG-specific Th1 and Th17 cells. B-cell depletion prevented or reversed established rMOG-induced EAE, which was associated with less central nervous system (CNS) inflammation, elimination of meningeal B cells, and reduction of MOG-specific Th1 and Th17 cells. In contrast, in MOG p35-55-induced EAE, B cells did not become activated or efficiently polarize proinflammatory MOG-specific T cells, similar to naive B cells. In this setting, anti-CD20 treatment exacerbated EAE, and did not impede development of Th1 or Th17 cells. Irrespective of the EAE model used, B-cell depletion reduced the frequency of CD4(+)CD25(+)Foxp3(+) regulatory T cells (Treg), and increased the proinflammatory polarizing capacity of remaining myeloid APCs.
Interpretation: Our study highlights distinct roles for B cells in CNS autoimmunity. Clinical benefit from anti-CD20 treatment may relate to inhibition of proinflammatory B cell APC function. In certain clinical settings, however, elimination of unactivated B cells, which participate in regulation of T cells and other APC, may be undesirable. Differences in immune responses to MOG protein and peptide may be important considerations when choosing an EAE model for testing novel B cell-targeting agents for MS.
Figures





References
-
- Prineas JW, Connell F. The fine structure of chronically active multiple sclerosis plaques. Neurology. 1978;28:68–75. - PubMed
-
- Genain CP, Cannella B, Hauser SL, Raine CS. Identification of autoantibodies associated with myelin damage in multiple sclerosis. Nat Med. 1999;5:170–175. - PubMed
-
- Keegan M, Konig F, McClelland R, et al. Relation between humoral pathological changes in multiple sclerosis and response to therapeutic plasma exchange. Lancet. 2005;366:579–582. - PubMed
-
- Constant S, Schweitzer N, West J, et al. B lymphocytes can be competent antigen-presenting cells for priming CD4+ T cells to protein antigens in vivo. J Immunol. 1995;155:3734–3741. - PubMed
-
- Constant S, Sant’Angelo D, Pasqualini T, et al. Peptide and protein antigens require distinct antigen-presenting cell subsets for the priming of CD4+ T cells. J Immunol. 1995;154:4915–4923. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

