Characterization of hyaluronan and TSG-6 in skin scarring: differential distribution in keloid scars, normal scars and unscarred skin
- PMID: 20642475
- PMCID: PMC3504979
- DOI: 10.1111/j.1468-3083.2010.03792.x
Characterization of hyaluronan and TSG-6 in skin scarring: differential distribution in keloid scars, normal scars and unscarred skin
Abstract
Background: Hyaluronan (HA) is a major component of the extracellular matrix (ECM) with increased synthesis during tissue repair. Tumour necrosis factor-stimulated gene-6 (TSG-6) is known to catalyze the covalent transfer of heavy chains (HC1 and HC2) from inter-α-inhibitor (IαI) onto HA, and resultant HC•HA complexes have been implicated in physiological and pathological processes related to remodelling and inflammation.
Objective: The aims of this study were to determine the expression of HA, TSG-6 and the IαI polypeptides in unscarred skin, normal scars and keloid scars.
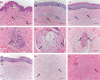
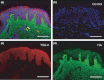
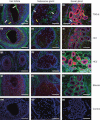
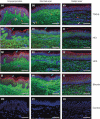
Methods: Formalin-fixed paraffin-embedded sections of unscarred skin, normal scars and keloid scars were prepared from patient samples collected during scar revision surgery. Haematoxylin and eosin, as well as immunofluorescent staining for HA, TSG-6 and the three polypeptide chains of IαI (i.e. HC1, HC2 and bikunin) were performed.
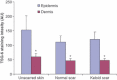
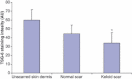
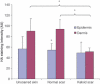
Results: All skin types stained positive for TSG-6, HC1, HC2 and bikunin, associated with keratinocytes, fibroblasts and skin appendages all in close proximity to HA. Keloid lesions showed altered HA organization patterns compared with unscarred skin and normal scars. TSG-6 staining was significantly more intense in the epidermis compared with the dermis of all sample types. There was a significant reduction in TSG-6 levels within keloid lesions compared with the dermis of unscarred skin (P=0.017).
Conclusion: TSG-6 is expressed in unscarred skin, where its close association with HA and IαI could give rise to TSG-6-mediated HC•HA formation within this tissue. A reduction in the beneficial effects of TSG-6, caused by diminished protein levels in keloid lesions, could contribute to this abnormal scarring process.
© 2010 The Authors. Journal of the European Academy of Dermatology and Venereology © 2010 European Academy of Dermatology and Venereology.
Figures

References
-
- Ferguson MWJ, Whitby DJ, Shah M, Armstrong J, Siebert JW, Longaker MT. Scar formation: the spectral nature of fetal and adult wound repair. Plast Reconstr Surg. 1996;97:854–860. - PubMed
-
- Marneros AG, Norris JEC, Watanabe S, Reichenberger E, Olsen BR. Genome scans provide evidence for keloid susceptibility loci on chromosomes 2q23 and 7p11. J Invest Dermatol. 2004;122:1126–1132. - PubMed
-
- Chen Y, Gao JH, Liu XJ, Yan X, Song M. Linkage analysis of keloid susceptibility loci on chromosome 7p11 in a Chinese pedigree. Nan fang yi ke da xue xue bao (Journal of Southern Medical University) 2006;26:623–637. - PubMed
-
- Bayat A, McGrouther DA. Clinical management of skin scarring. Skinmed. 2005;4:165–173. - PubMed
-
- Marneros AG, Krieg T. Keloids--clinical diagnosis, pathogenesis, and treatment options. J Dtsch Dermatol Ges. 2004;2:905–913. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

