Infection with the intracellular bacterium, Listeria monocytogenes, overrides established tolerance in a mouse cardiac allograft model
- PMID: 20642679
- PMCID: PMC4060596
- DOI: 10.1111/j.1600-6143.2010.03066.x
Infection with the intracellular bacterium, Listeria monocytogenes, overrides established tolerance in a mouse cardiac allograft model
Abstract
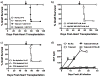
Infections and TLR signals at the time of transplantation have been shown to prevent the induction of tolerance, but their effect on allografts after tolerance has been established is unclear. We here report that infection with Listeria monocytogenes precipitated the loss of tolerance and the MyD88- and T cell-dependent rejection of accepted cardiac allografts in mice. This loss of tolerance was associated with increases in the numbers of graft-infiltrating macrophages and dendritic cells, as well as CD4(+)FoxP3(-) and CD8(+) T cells. Rejection was also associated with increased numbers of graft-infiltrating alloreactive as well as Listeria-reactive IFNgamma-producing T cells. Rejection of the established grafts required both IL-6 and IFNss, cytokines produced during acute Listeria infection. However, IL-6 and IFNss alone, even when present at higher concentrations than during Listeria infection, were insufficient to break tolerance, while the combination of IL-6 and IFNss was sufficient to break tolerance. These and in vitro observations that IL-6 but not IFNss enhanced T cell proliferation while IFNss but not IL-6 enhanced IFNgamma production support a hypothesis that these cytokines play nonredundant roles. In conclusion, these studies demonstrate that the proinflammatory effects of infections can induce the loss of tolerance and acute rejection of accepted allografts.
Figures


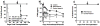
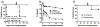
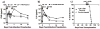
Comment in
-
Just how stable is allograft tolerance?Am J Transplant. 2010 Jul;10(7):1499-500. doi: 10.1111/j.1600-6143.2010.03171.x. Am J Transplant. 2010. PMID: 20642671 No abstract available.
References
-
- Bushell A, Morris PJ, Wood KJ. Transplantation tolerance induced by antigen pretreatment and depleting anti-CD4 antibody depends on CD4+ T cell regulation during the induction phase of the response. Eur J Immunol. 1995;25(9):2643–2649. - PubMed
-
- Cobbold SP, Adams E, Marshall SE, Davies JD, Waldmann H. Mechanisms of peripheral tolerance and suppression induced by monoclonal antibodies to CD4 and CD8. Immunol Rev. 1996;149:5–33. - PubMed
-
- Elster EA, Xu H, Tadaki DK, Montgomery S, Burkly LC, Berning JD, et al. Treatment with the humanized CD154-specific monoclonal antibody, hu5C8, prevents acute rejection of primary skin allografts in nonhuman primates. Transplantation. 2001;72(9):1473–1478. - PubMed
-
- Kawai T, Cosimi AB, Colvin RB, Powelson J, Eason J, Kozlowski T, et al. Mixed allogeneic chimerism and renal allograft tolerance in cynomolgus monkeys. Transplantation. 1995;59(2):256–262. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

