Liposomal encapsulation enhances and prolongs the anti-inflammatory effects of water-soluble dexamethasone phosphate in experimental adjuvant arthritis
- PMID: 20642832
- PMCID: PMC2945041
- DOI: 10.1186/ar3089
Liposomal encapsulation enhances and prolongs the anti-inflammatory effects of water-soluble dexamethasone phosphate in experimental adjuvant arthritis
Abstract
Introduction: The objective of this study was to evaluate the efficacy of intravenous (i.v.) injection of liposomally encapsulated dexamethasone phosphate (DxM-P) in comparison to free DxM-P in rats with established adjuvant arthritis (AA). This study focused on polyethylene glycol (PEG)-free liposomes, to minimize known allergic reactions caused by neutral PEG-modified (PEG-ylated) liposomes.
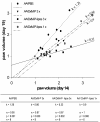
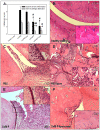
Methods: Efficacy was assessed clinically and histologically using standard scores. Non-specific and specific immune parameters were monitored. Activation of peritoneal macrophages was analyzed via cytokine profiling. Pharmacokinetics/biodistribution of DxM in plasma, synovial membrane, spleen and liver were assessed via mass spectrometry.
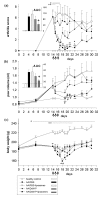
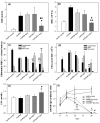
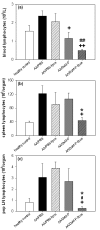
Results: Liposomal DxM-P (3 × 1 mg/kg body weight; administered intravenously (i.v.) on Days 14, 15 and 16 of AA) suppressed established AA, including histological signs, erythrocyte sedimentation rate, white blood cell count, circulating anti-mycobacterial IgG, and production of interleukin-1beta (IL-1β) and IL-6 by peritoneal macrophages. The suppression was strong and long-lasting. The clinical effects of liposomal DxM-P were dose-dependent for dosages between 0.01 and 1.0 mg/kg. Single administration of 1 mg/kg liposomal DxM-P and 3 × 1 mg/kg of free DxM-P showed comparable effects consisting of a partial and transient suppression. Moreover, the effects of medium-dose liposomal DxM-P (3 × 0.1 mg/kg) were equal (in the short term) or superior (in the long term) to those of high-dose free DxM-P (3 × 1 mg/kg), suggesting a potential dose reduction by a factor between 3 and 10 by liposomal encapsulation. For at least 48 hours after the last injection, the liposomal drug achieved significantly higher levels in plasma, synovial membrane, spleen and liver than the free drug.
Conclusions: This new PEG-free formulation of macrophage-targeting liposomal DxM-P considerably reduces the dose and/or frequency required to treat AA, with a potential to enhance or prolong therapeutic efficacy and limit side-effects also in the therapy of rheumatoid arthritis. Depot and/or recirculation effects in plasma, inflamed joint, liver, and spleen may contribute to this superiority of liposomally encapsulated DxM-P.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

