Electric field-driven disruption of a native beta-sheet protein conformation and generation of a helix-structure
- PMID: 20643079
- PMCID: PMC2905109
- DOI: 10.1016/j.bpj.2010.04.040
Electric field-driven disruption of a native beta-sheet protein conformation and generation of a helix-structure
Abstract
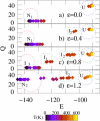
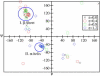
We demonstrate that an external constant electric field is able to modify the secondary structure of a protein and induce a transition from a beta-sheet into a helix-like conformation. This dramatic change is driven by a global rearrangement of the dipole moments at the amide planes. We also predict electric-field-induced modifications of the intermediate states of the protein.
Copyright (c) 2010 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures

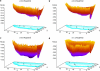
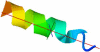
Similar articles
-
CD4 binding partially locks the bridging sheet in gp120 but leaves the beta2/3 strands flexible.J Mol Biol. 2005 Jul 15;350(3):514-27. doi: 10.1016/j.jmb.2005.05.009. J Mol Biol. 2005. PMID: 15946678
-
Local conformational stability of HIV-1 gp120 in unliganded and CD4-bound states as defined by amide hydrogen/deuterium exchange.J Virol. 2010 Oct;84(19):10311-21. doi: 10.1128/JVI.00688-10. Epub 2010 Jul 21. J Virol. 2010. PMID: 20660185 Free PMC article.
-
Secondary structure of gp160 and gp120 envelope glycoproteins of human immunodeficiency virus type 1: a Fourier transform infrared spectroscopic study.J Virol. 1993 Jun;67(6):3552-60. doi: 10.1128/JVI.67.6.3552-3560.1993. J Virol. 1993. PMID: 8497064 Free PMC article.
-
Pressure-induced transformation of alpha-helix to beta-sheet in the secondary structures of amyloid beta (1-40) peptide exacerbated by temperature.J Biomol Struct Dyn. 2002 Feb;19(4):619-25. doi: 10.1080/07391102.2002.10506768. J Biomol Struct Dyn. 2002. PMID: 11843623
-
HIV gp120: double lock strategy foils host defences.Structure. 1998 Aug 15;6(8):945-9. doi: 10.1016/s0969-2126(98)00096-3. Structure. 1998. PMID: 9739096 Review.
Cited by
-
Dramatic Differences between the Structural Susceptibility of the S1 Pre- and S2 Postfusion States of the SARS-CoV-2 Spike Protein to External Electric Fields Revealed by Molecular Dynamics Simulations.Viruses. 2023 Dec 11;15(12):2405. doi: 10.3390/v15122405. Viruses. 2023. PMID: 38140646 Free PMC article.
-
Electro-opening of a microtubule lattice in silico.Comput Struct Biotechnol J. 2021 Mar 4;19:1488-1496. doi: 10.1016/j.csbj.2021.02.007. eCollection 2021. Comput Struct Biotechnol J. 2021. PMID: 33815687 Free PMC article.
-
Molecular dynamics simulation of the nanosecond pulsed electric field effect on kinesin nanomotor.Sci Rep. 2019 Dec 23;9(1):19721. doi: 10.1038/s41598-019-56052-3. Sci Rep. 2019. PMID: 31873109 Free PMC article.
-
Analysing calcium signalling of cells under high shear flows using discontinuous dielectrophoresis.Sci Rep. 2015 Jul 23;5:11973. doi: 10.1038/srep11973. Sci Rep. 2015. PMID: 26202725 Free PMC article.
-
Nanosecond pulsed electric signals can affect electrostatic environment of proteins below the threshold of conformational effects: The case study of SOD1 with a molecular simulation study.PLoS One. 2019 Aug 27;14(8):e0221685. doi: 10.1371/journal.pone.0221685. eCollection 2019. PLoS One. 2019. PMID: 31454403 Free PMC article.
References
-
- Etienne M.A., Aucoin J.P., Hammer R.P. Stoichiometric inhibition of amyloid β-protein aggregation with peptides containing alternating α,α-disubstituted amino acids. J. Am. Chem. Soc. 2006;128:3522–3523. - PubMed
-
- Kelly J.W. The alternative conformations of amyloidogenic proteins and their multi-step assembly pathways. Curr. Opin. Struct. Biol. 1998;8:101–106. - PubMed
-
- Lynn D.G., Meredith S.C. Review: Model peptides and the physicochemical approach to β-amyloids. J. Struct. Biol. 2000;130:153–173. - PubMed
-
- Wada A. The α-helix as an electric macro-dipole. Adv. Biophys. 1976;9:1–63. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

