Electric field-driven disruption of a native beta-sheet protein conformation and generation of a helix-structure
- PMID: 20643079
- PMCID: PMC2905109
- DOI: 10.1016/j.bpj.2010.04.040
Electric field-driven disruption of a native beta-sheet protein conformation and generation of a helix-structure
Abstract
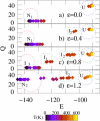
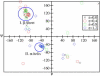
We demonstrate that an external constant electric field is able to modify the secondary structure of a protein and induce a transition from a beta-sheet into a helix-like conformation. This dramatic change is driven by a global rearrangement of the dipole moments at the amide planes. We also predict electric-field-induced modifications of the intermediate states of the protein.
Copyright (c) 2010 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures

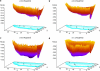
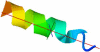
References
-
- Etienne M.A., Aucoin J.P., Hammer R.P. Stoichiometric inhibition of amyloid β-protein aggregation with peptides containing alternating α,α-disubstituted amino acids. J. Am. Chem. Soc. 2006;128:3522–3523. - PubMed
-
- Kelly J.W. The alternative conformations of amyloidogenic proteins and their multi-step assembly pathways. Curr. Opin. Struct. Biol. 1998;8:101–106. - PubMed
-
- Lynn D.G., Meredith S.C. Review: Model peptides and the physicochemical approach to β-amyloids. J. Struct. Biol. 2000;130:153–173. - PubMed
-
- Wada A. The α-helix as an electric macro-dipole. Adv. Biophys. 1976;9:1–63. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

