Toxicological and pathophysiological roles of reactive oxygen and nitrogen species
- PMID: 20643181
- PMCID: PMC8237863
- DOI: 10.1016/j.tox.2010.07.009
Toxicological and pathophysiological roles of reactive oxygen and nitrogen species
Erratum in
- Toxicology. 2011 Jan 11;279(1-3):208
Abstract
'Oxidative and Nitrative Stress in Toxicology and Disease' was the subject of a symposium held at the EUROTOX meeting in Dresden 15th September 2009. Reactive oxygen (ROS) and reactive nitrogen species (RNS) produced during tissue pathogenesis and in response to viral or chemical toxicants, induce a complex series of downstream adaptive and reparative events driven by the associated oxidative and nitrative stress. As highlighted by all the speakers, ROS and RNS can promote diverse biological responses associated with a spectrum of disorders including neurodegenerative/neuropsychiatric and cardiovascular diseases. Similar pathways are implicated during the process of liver and skin carcinogenesis. Mechanistically, reactive oxygen and nitrogen species drive sustained cell proliferation, cell death including both apoptosis and necrosis, formation of nuclear and mitochondrial DNA mutations, and in some cases stimulation of a pro-angiogenic environment. Here we illustrate the pivotal role played by oxidative and nitrative stress in cell death, inflammation and pain and its consequences for toxicology and disease pathogenesis. Examples are presented from five different perspectives ranging from in vitro model systems through to in vivo animal model systems and clinical outcomes.
Copyright 2010 Elsevier Ireland Ltd. All rights reserved.
Figures



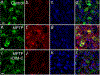
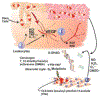

References
-
- Affara NI2004 and Robertson FM, Vascular endothelial growth factor as a survival factor in tumor-associated angiogenesis. In Vivo. 18(5) (2004), pp. 525–42. - PubMed
-
- Affara NI2006 Trempus CS, Schanbacher BL, Pei P, Mallery SR, Bauer JA and Robertson FM, Activation of Akt and mTOR in CD34+/K15+ keratinocyte stem cells and skin tumors during multi-stage mouse skin carcinogenesis. Anticancer Res. 26(4B) (2006), pp. 2805–20. - PubMed
-
- Ariza ME1996 Oberyszyn AS, Robertson FM and Williams MV, Mutagenic potential of peripheral blood leukocytes: in vivo exposure to the carcinogen 7,12 dimethylbenz[a]anthracene, and the tumor promoter 12-O-tetradecanoylphorbol acetate followed by in vitro co-culture with AS52 cells. Cancer Lett. 106(1) (1996) pp. 9–16. - PubMed
-
- Aslan M. 2008Cort A. and Yucel I, Oxidative and nitrative stress markers in glaucoma, Free Radical Biol. Med. 45 (2008), pp. 367–376. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

