Neogenin regulation of BMP-induced canonical Smad signaling and endochondral bone formation
- PMID: 20643353
- PMCID: PMC2924163
- DOI: 10.1016/j.devcel.2010.06.016
Neogenin regulation of BMP-induced canonical Smad signaling and endochondral bone formation
Abstract
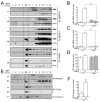
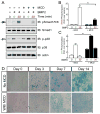
Neogenin has been identified as a receptor for the neuronal axon guidance cues netrins and RGMs (repulsive guidance molecules). Here we provide evidence for neogenin in regulating endochondral bone development and BMP (bone morphogenetic protein) signaling. Neogenin-deficient mice were impaired in digit/limb development and endochondral ossification. BMP2 induction of Smad1/5/8 phosphorylation and Runx2 expression, but not noncanonical p38 MAPK activation, was reduced in chondrocytes from neogenin mutant mice. BMP receptor association with membrane microdomains, which is necessary for BMP signaling to Smad, but not p38 MAPK, was diminished in neogenin-deficient chondrocytes. Furthermore, RGMs appear to mediate neogenin interaction with BMP receptors in chondrocytes. Taken together, our results indicate that neogenin promotes chondrogenesis in vitro and in vivo, revealing an unexpected mechanism underlying neogenin regulation of BMP signaling.
(c) 2010 Elsevier Inc. All rights reserved.
Figures

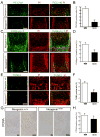
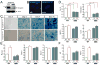
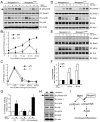
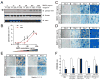

Comment in
-
A skeleton in the closet: neogenin guides bone development.Dev Cell. 2010 Jul 20;19(1):1-2. doi: 10.1016/j.devcel.2010.07.004. Dev Cell. 2010. PMID: 20643341
References
-
- Babitt JL, Huang FW, Wrighting DM, Xia Y, Sidis Y, Samad TA, Campagna JA, Chung RT, Schneyer AL, Woolf CJ, et al. Bone morphogenetic protein signaling by hemojuvelin regulates hepcidin expression. Nature genetics. 2006;38:531–539. - PubMed
-
- Babitt JL, Zhang Y, Samad TA, Xia Y, Tang J, Campagna JA, Schneyer AL, Woolf CJ, Lin HY. Repulsive guidance molecule (RGMa), a DRAGON homologue, is a bone morphogenetic protein co-receptor. The Journal of biological chemistry. 2005;280:29820–29827. - PubMed
-
- Baur ST, Mai JJ, Dymecki SM. Combinatorial signaling through BMP receptor IB and GDF5: shaping of the distal mouse limb and the genetics of distal limb diversity. Development (Cambridge, England) 2000;127:605–619. - PubMed
-
- Cole SJ, Bradford D, Cooper HM. Neogenin: A multi-functional receptor regulating diverse developmental processes. The international journal of biochemistry & cell biology. 2007;39:1569–1575. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

