Epidermal wound repair is regulated by the planar cell polarity signaling pathway
- PMID: 20643356
- PMCID: PMC2965174
- DOI: 10.1016/j.devcel.2010.06.008
Epidermal wound repair is regulated by the planar cell polarity signaling pathway
Erratum in
- Dev Cell. 2010 Aug 17;19(2):353. Parekh, Vishwas [added]
Abstract
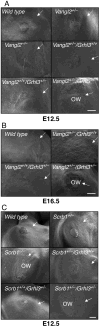
The mammalian PCP pathway regulates diverse developmental processes requiring coordinated cellular movement, including neural tube closure and cochlear stereociliary orientation. Here, we show that epidermal wound repair is regulated by PCP signaling. Mice carrying mutant alleles of PCP genes Vangl2, Celsr1, PTK7, and Scrb1, and the transcription factor Grhl3, interact genetically, exhibiting failed wound healing, neural tube defects, and disordered cochlear polarity. Using phylogenetic analysis, ChIP, and gene expression in Grhl3(-)(/-) mice, we identified RhoGEF19, a homolog of a RhoA activator involved in PCP signaling in Xenopus, as a direct target of GRHL3. Knockdown of Grhl3 or RhoGEF19 in keratinocytes induced defects in actin polymerization, cellular polarity, and wound healing, and re-expression of RhoGEF19 rescued these defects in Grhl3-kd cells. These results define a role for Grhl3 in PCP signaling and broadly implicate this pathway in epidermal repair.
(c) 2010 Elsevier Inc. All rights reserved.
Figures



References
-
- Adler PN. Planar signaling and morphogenesis in Drosophila. Dev Cell. 2002;2:525–535. - PubMed
-
- Blair A, Tomlinson A, Pham H, Gunsalus KC, Goldberg ML, Laski FA. Twinstar, the Drosophila homolog of cofilin/ADF, is required for planar cell polarity patterning. Development. 2006;133:1789–1797. - PubMed
-
- Curtin JA, Quint E, Tsipouri V, Arkell RM, Cattanach B, Copp AJ, Henderson DJ, Spurr N, Stanier P, Fisher EM, et al. Mutation of Celsr1 disrupts planar polarity of inner ear hair cells and causes severe neural tube defects in the mouse. Curr Biol. 2003;13:1129–1133. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

