Participation of older patients with prostate cancer in Medicare eligible trials
- PMID: 20643449
- PMCID: PMC3811922
- DOI: 10.1016/j.juro.2010.04.076
Participation of older patients with prostate cancer in Medicare eligible trials
Abstract
Purpose: On June 7, 2000 President Clinton issued an executive memorandum directing Medicare payment for routine patient care in qualifying clinical trials. We estimated the proportion of older patients with prostate cancer who were examined as part of a qualifying clinical trial, and the association between participation and patient characteristics.
Materials and methods: We performed an observational study using the Surveillance, Epidemiology and End Results Medicare database to determine participation in qualifying clinical trials in a sample of 37,216 men 66 years old or older who were enrolled in Medicare and diagnosed with prostate cancer between September 2000 and December 2002.
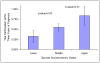
Results: Within 3 years of diagnosis 211 men (0.567%) received routine patient care in a qualifying clinical trial. These participants were more likely to be younger than 70 years (OR 1.687, 95% CI 1.27-2.24) and less likely to be less educated and reside in low income, metropolitan neighborhoods. White men were more likely to participate in clinical trials than nonwhite men but this association was not statistically significant (OR 1.426, CI 0.97-2.09). Participation varied significantly by registry site (0% to 1.2%) but not by tumor grade or stage, or prostate specific antigen status.
Conclusions: Few older patients with prostate cancer participated in qualifying trials between 2000 and 2002. Those who participated were not representative of the general population of older patients with prostate cancer. Greater efforts are required to expand trial enrollment and decrease disparities in research participation.
2010 American Urological Association Education and Research, Inc. Published by Elsevier Inc. All rights reserved.
Figures
References
-
- Catania C, De Pas T, Goldhirsch A, et al. Participation in clinical trials as viewed by the patient: understanding cultural and emotional aspects which influence choice. Oncology. 2008;74:177. - PubMed
-
- Lara PN, Jr, Higdon R, Lim N, et al. Prospective evaluation of cancer clinical trial accrual patterns: identifying potential barriers to enrollment. J Clin Oncol. 2001;19:1728. - PubMed
-
- Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: race-, sex-, and age-based disparities. JAMA. 2004;291:2720. - PubMed
-
- Outlaw FH, Bourjolly JN, Barg FK. A study on recruitment of black Americans into clinical trials through a cultural competence lens. Cancer Nurs. 2000;23:444. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical