LOGISMOS--layered optimal graph image segmentation of multiple objects and surfaces: cartilage segmentation in the knee joint
- PMID: 20643602
- PMCID: PMC3131162
- DOI: 10.1109/TMI.2010.2058861
LOGISMOS--layered optimal graph image segmentation of multiple objects and surfaces: cartilage segmentation in the knee joint
Abstract
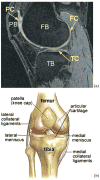
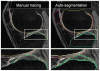
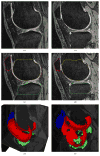
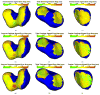
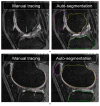
A novel method for simultaneous segmentation of multiple interacting surfaces belonging to multiple interacting objects, called LOGISMOS (layered optimal graph image segmentation of multiple objects and surfaces), is reported. The approach is based on the algorithmic incorporation of multiple spatial inter-relationships in a single n-dimensional graph, followed by graph optimization that yields a globally optimal solution. The LOGISMOS method's utility and performance are demonstrated on a bone and cartilage segmentation task in the human knee joint. Although trained on only a relatively small number of nine example images, this system achieved good performance. Judged by dice similarity coefficients (DSC) using a leave-one-out test, DSC values of 0.84 ± 0.04, 0.80 ± 0.04 and 0.80 ± 0.04 were obtained for the femoral, tibial, and patellar cartilage regions, respectively. These are excellent DSC values, considering the narrow-sheet character of the cartilage regions. Similarly, low signed mean cartilage thickness errors were obtained when compared to a manually-traced independent standard in 60 randomly selected 3-D MR image datasets from the Osteoarthritis Initiative database-0.11 ± 0.24, 0.05 ± 0.23, and 0.03 ± 0.17 mm for the femoral, tibial, and patellar cartilage thickness, respectively. The average signed surface positioning errors for the six detected surfaces ranged from 0.04 ± 0.12 mm to 0.16 ± 0.22 mm. The reported LOGISMOS framework provides robust and accurate segmentation of the knee joint bone and cartilage surfaces of the femur, tibia, and patella. As a general segmentation tool, the developed framework can be applied to a broad range of multiobject multisurface segmentation problems.
Figures
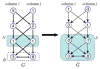
 in G problem into finding nonempty closed set
in G problem into finding nonempty closed set
 in G̃ with the same weight. Here K = 4.
in G̃ with the same weight. Here K = 4.


References
-
- Sonka M, Hlavac V, Boyle R. Image Processing, Analysis, and Machine Vision. 3. Mobile, AL: Thomson Eng; 2008.
-
- Wu X, Chen DZ. Optimal net surface problem with applications. Proc. 29th Int. Coll. Automata, Lang. Program. (ICALP); Jul. 2002; pp. 1029–1042.
-
- Tsai A, Wells W, Tempany C, Grimson E, Willsky A. Mutual information in coupled multi-shape model for medical image segmentation. Med Image Anal. 2004;8:429–445. - PubMed
-
- Litvin A, Karl WC. Coupled shape distribution-based segmentation of multiple objects. Inf Process Med Imag. 2005:345–356. - PubMed
Publication types
MeSH terms
Grants and funding
- N01-AR-2-2261/AR/NIAMS NIH HHS/United States
- N01-AR-2-2260/AR/NIAMS NIH HHS/United States
- P50 AR055533/AR/NIAMS NIH HHS/United States
- N01 AR022261/AR/NIAMS NIH HHS/United States
- N01-AR-2-2259/AR/NIAMS NIH HHS/United States
- N01 AR022259/AR/NIAMS NIH HHS/United States
- R01-EB004640/EB/NIBIB NIH HHS/United States
- N01 AR022262/AR/NIAMS NIH HHS/United States
- N01 AR022260/AR/NIAMS NIH HHS/United States
- N01-AR-2-2258/AR/NIAMS NIH HHS/United States
- K25 CA123112/CA/NCI NIH HHS/United States
- R44-AR052983/AR/NIAMS NIH HHS/United States
- R01 EB004640/EB/NIBIB NIH HHS/United States
- N01-AR-2-2262/AR/NIAMS NIH HHS/United States
- N01 AR022258/AR/NIAMS NIH HHS/United States
- R44 AR052983/AR/NIAMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

