Estimating the risk of HIV transmission from homosexual men receiving treatment to their HIV-uninfected partners
- PMID: 20643658
- PMCID: PMC3252623
- DOI: 10.1136/sti.2010.042622
Estimating the risk of HIV transmission from homosexual men receiving treatment to their HIV-uninfected partners
Abstract
Objective: To determine how the risk of HIV transmission from homosexual men receiving antiretroviral treatment is related to patterns of patient monitoring and condom use.
Methods: A stochastic mathematical simulation model was developed of cohorts of men in the Netherlands who have sex with men (MSM), defining the parameters of the model using observational cohort data. The model incorporates viral load trends during first-line treatment, patient monitoring and different scenarios for the way in which condom use may depend on recent viral load measurements. The model does not include the effect of sexually transmitted infections on HIV transmission.
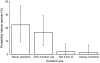
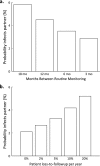
Results: For MSM receiving treatment, the risk of transmitting HIV to their long-term partner is 22% (uncertainty interval: 9-37%) if condoms are never used. With incomplete use (in 30% of sex acts) the risk is reduced slightly, to 17% (7-29%). However, the risk is as low as 3% (0.2-8%) when men receiving treatment use condoms only 6 months beyond their last undetectable viral load measurement. The risk is further reduced when 3 months is the time period beyond which condoms are used.
Conclusions: When condom use by HIV-infected men receiving combination treatment with antiretroviral agents is based on their last viral load measurement, the transmission risk is much lower than with incomplete condom use. The key message for patients is that although always using condoms during treatment is the best way to protect partners from the risk of HIV transmission, when such use cannot be achieved, the second best strategy is to use condoms whenever the last undetectable viral load was measured more than 3 months ago.
Conflict of interest statement
Figures

References
-
- Hammer SM, Squires KE, Hughes MD, et al. A controlled trial of two nucleoside analogues plus indinavir in persons with human immunodeficiency virus infection and CD4 cell counts of 200 per cubic millimeter or less. AIDS Clinical Trials Group 320 Study Team. N Engl J Med 1997;337:725–33 - PubMed
-
- Staszewski S, Morales-Ramirez J, Tashima KT, et al. Efavirenz plus zidovudine and lamivudine, efavirenz plus indinavir and indinavir plus zidovudine and lamivudine in the treatment of HIV-1 infection in adults. Study 006 Team. N Engl J Med 1999;341:1865–73 - PubMed
-
- UNAIDS Report on the Global AIDS epidemic, http://www.unaids.org/en/knowledge/HIVdata/globalreport/2008/2008_global..., 2008
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
