Nitric oxide and AMPK cooperatively regulate PGC-1 in skeletal muscle cells
- PMID: 20643772
- PMCID: PMC2988518
- DOI: 10.1113/jphysiol.2010.194035
Nitric oxide and AMPK cooperatively regulate PGC-1 in skeletal muscle cells
Abstract
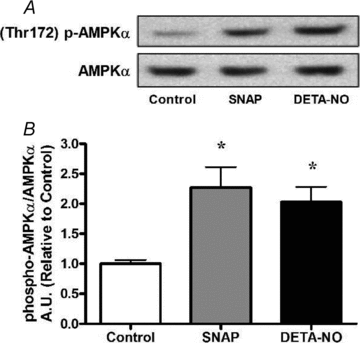
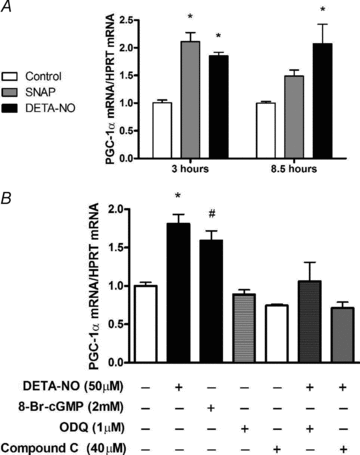
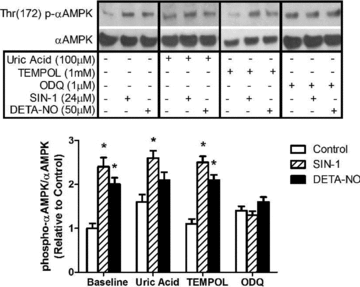
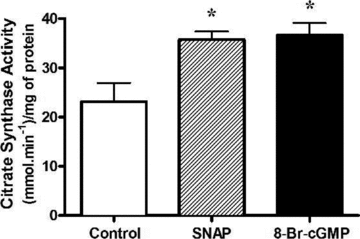
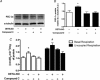
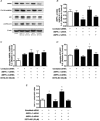
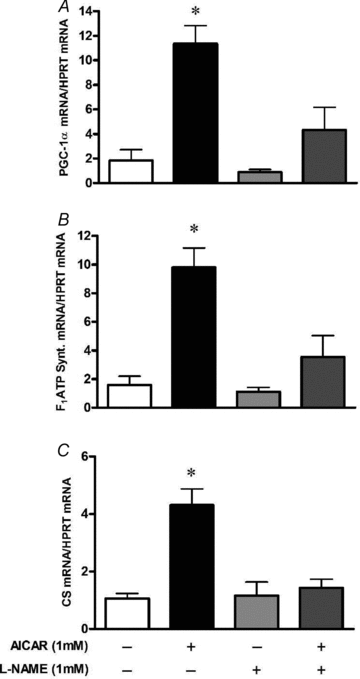
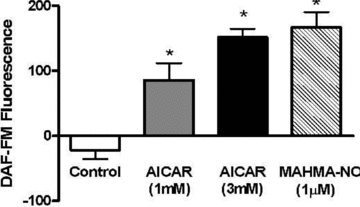
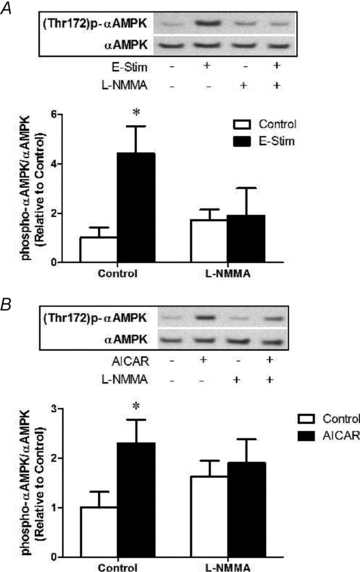
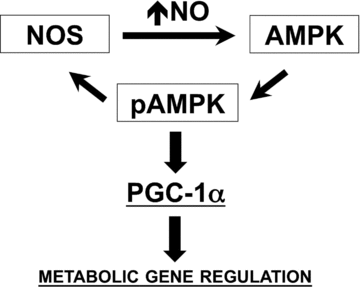
Nitric oxide (NO) induces mitochondrial biogenesis in skeletal muscle cells via upregulation of the peroxisome proliferator-activated receptor-γ coactivator 1α (PGC-1α). Further, we have shown that nitric oxide interacts with the metabolic sensor enzyme, AMPK. Therefore, we tested the hypothesis that nitric oxide and AMPK act synergistically to upregulate PGC-1α mRNA expression and stimulate mitochondrial biogenesis in culture. L6 myotubes treated with nitric oxide donors, S-nitroso-N-penicillamine (SNAP, 25 μM) or diethylenetriamine-NONO (DETA-NO, 50 μM), exhibited elevated AMPK phosphorylation, PGC-1α mRNA and protein, and basal and uncoupled mitochondrial respiration (P < 0.05). Pre-treatment of cultures with the AMPK inhibitor, Compound C, prevented these effects. Knockdown of AMPKα1 in L6 myotubes using siRNA reduced AMPKα protein content and prevented upregulation of PGC-1α mRNA by DETA-NO. Meanwhile, siRNA knockdown of AMPKα2 had no effect on total AMPKα protein content or PGC-1α mRNA. These results suggest that NO effects on PGC-1α expression are mediated by AMPKα1. Paradoxically, we found that the AMPK-activating compound, AICAR, induced NO release from L6 myotubes, and that AICAR-induced upregulation of PGC-1α mRNA was prevented by inhibition of NOS with N(G)-nitro-L-arginine methyl ester (L-NAME, 1 mM). Additionally, incubation of isolated mouse extensor digitorum longus (EDL) muscles with 2 mM AICAR for 20 min or electrical stimulation (10 Hz, 13 V) for 10 min induced phosphorylation of AMPKα (P < 0.05), which was completely prevented by pre-treatment with the NOS inhibitor, L-N(G)-monomethyl arginine (L-NMMA, 1 mM). These data identify the AMPKα1 isoform as the mediator of NO-induced effects in skeletal muscle cells. Further, this study supports a proposed model of synergistic interaction between AMPK and NOS that is critical for maintenance of metabolic function in skeletal muscle cells.
Figures
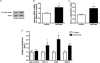
References
-
- Akimoto T, Sorg BS, Yan Z. Real-time imaging of peroxisome proliferator-activated receptor-γ coactivator-1α promoter activity in skeletal muscles of living mice. Am J Physiol Cell Physiol. 2004;287:C790–C796. - PubMed
-
- Balon TW, Jasman AP. Acute exposure to AICAR increases glucose transport in mouse EDL and soleus muscle. Biochem Biophys Res Commun. 2001;282:1008–1011. - PubMed
-
- Bonen A, Clark MG, Henriksen EJ. Experimental approaches in muscle metabolism: hindlimb perfusion and isolated muscle incubations. Am J Physiol Endocrinol Metab. 1994;266:E1–E16. - PubMed
-
- Bonnard C, Durand A, Vidal H, Rieusset J. Changes in adiponectin, its receptors and AMPK activity in tissues of diet-induced diabetic mice. Diabetes Metab. 2008;34:52–61. - PubMed
-
- Borniquel S, Valle I, Cadenas S, Lamas S, Monsalve M. Nitric oxide regulates mitochondrial oxidative stress via the transcriptional coactivator PGC1α. FASEB J. 2006;20:1889–1891. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

