A 9 bp cis-element in the promoters of class I small heat shock protein genes on chromosome 3 in rice mediates L-azetidine-2-carboxylic acid and heat shock responses
- PMID: 20643810
- PMCID: PMC2955743
- DOI: 10.1093/jxb/erq230
A 9 bp cis-element in the promoters of class I small heat shock protein genes on chromosome 3 in rice mediates L-azetidine-2-carboxylic acid and heat shock responses
Abstract
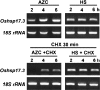
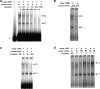
In rice, the class I small heat shock protein (sHSP-CI) genes were found to be selectively induced by L-azetidine-2-carboxylic acid (AZC) on chromosome 3 but not chromosome 1. Here it is shown that a novel cis-responsive element contributed to the differential regulation. By serial deletion and computational analysis, a 9 bp putative AZC-responsive element (AZRE), GTCCTGGAC, located between nucleotides -186 and -178 relative to the transcription initiation site of Oshsp17.3 was revealed. Deletion of this putative AZRE from the promoter abolished its ability to be induced by AZC. Moreover, electrophoretic mobility shift assay (EMSA) revealed that the AZRE interacted specifically with nuclear proteins from AZC-treated rice seedlings. Two AZRE-protein complexes were detected by EMSA, one of which could be competed out by a canonical heat shock element (HSE). Deletion of the AZRE also affected the HS response. Furthermore, transient co-expression of the heat shock factor OsHsfA4b with the AZRE in the promoter of Oshsp17.3 was effective. The requirement for the putative AZRE for AZC and HS responses in transgenic Arabidopsis was also shown. Thus, AZRE represents an alternative form of heat HSE, and its interaction with canonical HSEs through heat shock factors may be required to respond to HS and AZC.
Figures

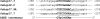



Similar articles
-
Characterization of the genomic structures and selective expression profiles of nine class I small heat shock protein genes clustered on two chromosomes in rice (Oryza sativa L.).Plant Mol Biol. 2004 Nov;56(5):795-809. doi: 10.1007/s11103-004-5182-z. Epub 2005 Mar 24. Plant Mol Biol. 2004. PMID: 15803416
-
Silencing of class I small heat shock proteins affects seed-related attributes and thermotolerance in rice seedlings.Planta. 2019 Dec 3;251(1):26. doi: 10.1007/s00425-019-03318-9. Planta. 2019. PMID: 31797121
-
Expression and promoter analysis of the OsHSP16.9C gene in rice.Biochem Biophys Res Commun. 2016 Oct 14;479(2):260-265. doi: 10.1016/j.bbrc.2016.09.056. Epub 2016 Sep 14. Biochem Biophys Res Commun. 2016. PMID: 27639642
-
A proximal promoter region of Arabidopsis DREB2C confers tissue-specific expression under heat stress.J Integr Plant Biol. 2012 Sep;54(9):640-51. doi: 10.1111/j.1744-7909.2012.01137.x. Epub 2012 Aug 30. J Integr Plant Biol. 2012. PMID: 22716647
-
Proteomics of rice in response to heat stress and advances in genetic engineering for heat tolerance in rice.Plant Cell Rep. 2011 Dec;30(12):2155-65. doi: 10.1007/s00299-011-1122-y. Epub 2011 Jul 17. Plant Cell Rep. 2011. PMID: 21769604 Review.
Cited by
-
Comparative transcriptome analysis of panicle development under heat stress in two rice (Oryza sativa L.) cultivars differing in heat tolerance.PeerJ. 2019 Aug 29;7:e7595. doi: 10.7717/peerj.7595. eCollection 2019. PeerJ. 2019. PMID: 31528506 Free PMC article.
-
OsMLP423 Is a Positive Regulator of Tolerance to Drought and Salt Stresses in Rice.Plants (Basel). 2022 Jun 23;11(13):1653. doi: 10.3390/plants11131653. Plants (Basel). 2022. PMID: 35807608 Free PMC article.
-
Regulatory motifs found in the small heat shock protein (sHSP) gene family in tomato.BMC Genomics. 2018 Dec 11;19(Suppl 8):860. doi: 10.1186/s12864-018-5190-z. BMC Genomics. 2018. PMID: 30537925 Free PMC article.
-
Using RNA-seq to Profile Gene Expression of Spikelet Development in Response to Temperature and Nitrogen during Meiosis in Rice (Oryza sativa L.).PLoS One. 2015 Dec 29;10(12):e0145532. doi: 10.1371/journal.pone.0145532. eCollection 2015. PLoS One. 2015. PMID: 26714321 Free PMC article.
-
The role of promoter cis-element, mRNA capping, and ROS in the repression and salt-inducible expression of AtSOT12 in Arabidopsis.Front Plant Sci. 2015 Nov 6;6:974. doi: 10.3389/fpls.2015.00974. eCollection 2015. Front Plant Sci. 2015. PMID: 26594223 Free PMC article.
References
-
- Almoguera C, Rojas A, Diaz-Martin J, Prieto-Dapena P, Carranco R, Jordano J. A seed-specific heat-shock transcription factor involved in developmental regulation during embryogenesis in sunflower. Journal of Biological Chemistry. 2002;277:43866–43872. - PubMed
-
- Banzet N, Richaud C, Deveaux Y, Kazmaier M, Gagnon J, Triantaphylidès C. Accumulation of small heat shock proteins, including mitochondrial HSP22, induced by oxidative stress and adaptive response in tomato cells. The Plant Journal. 1998;13:519–527. - PubMed
-
- Christensen AH, Quail PH. Ubiquitin promoter-based vectors for high-level expression of selectable and/or screenable marker genes in monocotyledonous plants. Transgenic Research. 1996;5:213–218. - PubMed

