Trabectedin plus pegylated liposomal doxorubicin in relapsed ovarian cancer: outcomes in the partially platinum-sensitive (platinum-free interval 6-12 months) subpopulation of OVA-301 phase III randomized trial
- PMID: 20643862
- PMCID: PMC3003616
- DOI: 10.1093/annonc/mdq352
Trabectedin plus pegylated liposomal doxorubicin in relapsed ovarian cancer: outcomes in the partially platinum-sensitive (platinum-free interval 6-12 months) subpopulation of OVA-301 phase III randomized trial
Abstract
Background: OVA-301 is a large randomized trial that showed superiority of trabectedin plus pegylated liposomal doxorubicin (PLD) over PLD alone in relapsed ovarian cancer. The optimal management of patients with partially platinum-sensitive relapse [6-12 months platinum-free interval (PFI)] is unclear.
Patients and methods: within OVA-301, we therefore now report on the outcomes for the 214 cases in this subgroup.
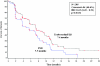
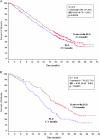
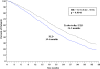
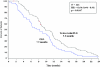
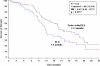
Results: Trabectedin/PLD resulted in a 35% risk reduction of disease progression (DP) or death [hazard ratio (HR) = 0.65, 95% confidence interval (CI), 0.45-0.92; P = 0.0152; median progression-free survival (PFS) 7.4 versus 5.5 months], and a significant 41% decrease in the risk of death (HR = 0.59; 95% CI, 0.43-0.82; P = 0.0015; median survival 23.0 versus 17.1 months). The safety of trabectedin/PLD in this subset mimicked that of the overall population. Similar proportions of patients received subsequent therapy in each arm (76% versus 77%), although patients in the trabectedin/PLD arm had a slightly lower proportion of further platinum (49% versus 55%). Importantly, patients in the trabectedin/PLD arm survived significantly longer after subsequent platinum (HR = 0.63; P = 0.0357; median 13.3 versus 9.8 months).
Conclusion: This hypothesis-generating analysis demonstrates that superior benefits with trabectedin/PLD in terms of PFS and survival in the overall population appear particularly enhanced in patients with partially sensitive disease (PFI 6-12 months).
Figures
Comment in
-
Trabectedin in ovarian cancer: could we expect more?Ann Oncol. 2011 Jan;22(1):7-8. doi: 10.1093/annonc/mdq641. Epub 2010 Nov 8. Ann Oncol. 2011. PMID: 21059638 No abstract available.
References
-
- Sessa C, De Braud F, Perotti A, et al. Trabectedin for women with ovarian carcinoma after treatment with platinum and taxanes fails. J Clin Oncol. 2005;23:1867–1874. - PubMed
-
- Del Campo JM, Roszak A, Bidzinski M, et al. Phase II randomized study of trabectedin given as two different every 3 weeks dose schedules (1.5 mg/m2 24 h or 1.3 mg/m2 3 h) to patients with relapsed, platinum-sensitive, advanced ovarian cancer. Ann Oncol. 2009;20:1794–1802. - PubMed
-
- Takahashi N, Li WW, Banerjee D, et al. Sequence-dependent enhancement of cytotoxicity produced by ecteinascidin 743 (ET-743) with doxorubicin or paclitaxel in soft tissue sarcoma cells. Clin Cancer Res. 2001;7:3251–3257. - PubMed
-
- Meco D, Colombo T, Ubezio P, et al. Effective combination of ET-743 and doxorubicin in sarcoma: preclinical studies. Cancer Chemother Pharmacol. 2003;52:131–138. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

