Epigenetic centromere specification directs aurora B accumulation but is insufficient to efficiently correct mitotic errors
- PMID: 20643881
- PMCID: PMC2930274
- DOI: 10.1083/jcb.201001035
Epigenetic centromere specification directs aurora B accumulation but is insufficient to efficiently correct mitotic errors
Abstract
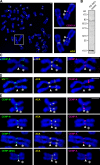
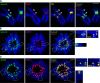
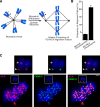
The nearly ubiquitous presence of repetitive centromere DNA sequences across eukaryotic species is in paradoxical contrast to their apparent functional dispensability. Centromeric chromatin is spatially delineated into the kinetochore-forming array of centromere protein A (CENP-A)-containing nucleosomes and the inner centromeric heterochromatin that lacks CENP-A but recruits the aurora B kinase that is necessary for correcting erroneous attachments to the mitotic spindle. We found that the self-perpetuating network of CENPs at the foundation of the kinetochore is intact at a human neocentromere lacking repetitive alpha-satellite DNA. However, aurora B is inappropriately silenced as a consequence of the altered geometry of the neocentromere, thereby compromising the error correction mechanism. This suggests a model wherein the neocentromere represents a primordial inheritance locus that requires subsequent generation of a robust inner centromere compartment to enhance fidelity of chromosome transmission.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

