Targeting of eEF1A with Amaryllidaceae isocarbostyrils as a strategy to combat melanomas
- PMID: 20643906
- PMCID: PMC3229423
- DOI: 10.1096/fj.10-162263
Targeting of eEF1A with Amaryllidaceae isocarbostyrils as a strategy to combat melanomas
Erratum in
-
ERRATUM.FASEB J. 2015 Sep;29(9):4080. doi: 10.1096/fj.10-162263ERR. FASEB J. 2015. PMID: 26330559 Free PMC article. No abstract available.
Abstract
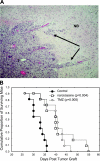
Melanomas display poor response rates to adjuvant therapies because of their intrinsic resistance to proapoptotic stimuli. This study indicates that such resistance can be overcome, at least partly, through the targeting of eEF1A elongation factor with narciclasine, an Amaryllidaceae isocarbostyril controlling plant growth. Narciclasine displays IC(50) growth inhibitory values between 30-100 nM in melanoma cell lines, irrespective of their levels of resistance to proapoptotic stimuli. Normal noncancerous cell lines are much less affected. At nontoxic doses, narciclasine also significantly improves (P=0.004) the survival of mice bearing metastatic apoptosis-resistant melanoma xenografts in their brain. The eEF1A targeting with narciclasine (50 nM) leads to 1) marked actin cytoskeleton disorganization, resulting in cytokinesis impairment, and 2) protein synthesis impairment (elongation and initiation steps), whereas apoptosis is induced at higher doses only (≥200 nM). In addition to molecular docking validation and identification of potential binding sites, we biochemically confirmed that narciclasine directly binds to human recombinant and yeast-purified eEF1A in a nanomolar range, but not to actin or elongation factor 2, and that 5 nM narciclasine is sufficient to impair eEF1A-related actin bundling activity. eEF1A is thus a potential target to combat melanomas regardless of their apoptosis-sensitivity, and this finding reconciles the pleiotropic cytostatic of narciclasine. -
Figures
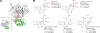


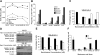

References
-
- Hamm C., Verma S., Petrella T., Bak K., Charette M. (2008) Melanoma Disease Site Group of Cancer Care Ontario's Program in evidence-based care. Biochemotherapy for the treatment of metastatic malignant melanoma: a systematic review. Cancer Treat. Rev. 34, 145– 146 - PubMed
-
- Atallah E., Flaherty L. (2005) Treatment of metastatic malignant melanoma. Curr. Treat. Options Oncol. 6, 185– 193 - PubMed
-
- Soengas M. S., Lowe S. W. (2003) Apoptosis and melanoma chemoresistance. Oncogene 22, 3138– 3151 - PubMed
-
- Eberle J., Kurbanov B. M., Hossini A. M., Trefzer U., Fecker L. F. (2007) Overcoming apoptosis deficiency of melanoma-hope for new therapeutic approaches. Drug Resist. Updat. 10, 218– 234 - PubMed
-
- Ivanov V. N., Bhoumik A., Ronai Z. (2003) Death receptors and melanoma resistance to apoptosis. Oncogene 22, 3152– 3161 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

