Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women
- PMID: 20643915
- PMCID: PMC3001187
- DOI: 10.1126/science.1193748
Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women
Erratum in
- Science. 2011 Jul 29;333(6042):524
Abstract
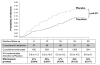
The Centre for the AIDS Program of Research in South Africa (CAPRISA) 004 trial assessed the effectiveness and safety of a 1% vaginal gel formulation of tenofovir, a nucleotide reverse transcriptase inhibitor, for the prevention of HIV acquisition in women. A double-blind, randomized controlled trial was conducted comparing tenofovir gel (n = 445 women) with placebo gel (n = 444 women) in sexually active, HIV-uninfected 18- to 40-year-old women in urban and rural KwaZulu-Natal, South Africa. HIV serostatus, safety, sexual behavior, and gel and condom use were assessed at monthly follow-up visits for 30 months. HIV incidence in the tenofovir gel arm was 5.6 per 100 women-years (person time of study observation) (38 out of 680.6 women-years) compared with 9.1 per 100 women-years (60 out of 660.7 women-years) in the placebo gel arm (incidence rate ratio = 0.61; P = 0.017). In high adherers (gel adherence > 80%), HIV incidence was 54% lower (P = 0.025) in the tenofovir gel arm. In intermediate adherers (gel adherence 50 to 80%) and low adherers (gel adherence < 50%), the HIV incidence reduction was 38 and 28%, respectively. Tenofovir gel reduced HIV acquisition by an estimated 39% overall, and by 54% in women with high gel adherence. No increase in the overall adverse event rates was observed. There were no changes in viral load and no tenofovir resistance in HIV seroconverters. Tenofovir gel could potentially fill an important HIV prevention gap, especially for women unable to successfully negotiate mutual monogamy or condom use.
Figures


Comment in
-
HIV prevention in women: next steps.Science. 2011 Jan 21;331(6015):284. doi: 10.1126/science.331.6015.284-a. Science. 2011. PMID: 21252332 No abstract available.
-
Re.: Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women.J Urol. 2011 May;185(5):1729. doi: 10.1016/S0022-5347(11)60197-3. J Urol. 2011. PMID: 22088702 No abstract available.
-
Pre-exposure prophylaxis for the prevention of HIV infection: a new prevention paradigm?Farm Hosp. 2016 May 1;40(3):219-24. doi: 10.7399/fh.2016.40.3.10439. Farm Hosp. 2016. PMID: 27145390 English.
References
-
- UNAIDS. WHO . AIDS Epidemic Update. Joint United Nations Programme on HIV/AIDS and World Health Organisation; Geneva: 2009.
-
- Bailey RC, et al. Male circumcision for HIV prevention in young men in Kisumu, Kenya: a randomised controlled trial. Lancet. 2007 Feb 24;369:643–656. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

