NMDA receptor-dependent GABAB receptor internalization via CaMKII phosphorylation of serine 867 in GABAB1
- PMID: 20643921
- PMCID: PMC2922270
- DOI: 10.1073/pnas.1000909107
NMDA receptor-dependent GABAB receptor internalization via CaMKII phosphorylation of serine 867 in GABAB1
Abstract
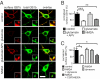
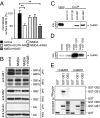
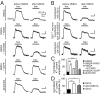
GABAB receptors are the G-protein-coupled receptors for GABA, the main inhibitory neurotransmitter in the brain. GABAB receptors are abundant on dendritic spines, where they dampen postsynaptic excitability and inhibit Ca2+ influx through NMDA receptors when activated by spillover of GABA from neighboring GABAergic terminals. Here, we show that an excitatory signaling cascade enables spines to counteract this GABAB-mediated inhibition. We found that NMDA application to cultured hippocampal neurons promotes dynamin-dependent endocytosis of GABAB receptors. NMDA-dependent internalization of GABAB receptors requires activation of Ca2+/Calmodulin-dependent protein kinase II (CaMKII), which associates with GABAB receptors in vivo and phosphorylates serine 867 (S867) in the intracellular C terminus of the GABAB1 subunit. Blockade of either CaMKII or phosphorylation of S867 renders GABAB receptors refractory to NMDA-mediated internalization. Time-lapse two-photon imaging of organotypic hippocampal slices reveals that activation of NMDA receptors removes GABAB receptors within minutes from the surface of dendritic spines and shafts. NMDA-dependent S867 phosphorylation and internalization is predominantly detectable with the GABAB1b subunit isoform, which is the isoform that clusters with inhibitory effector K+ channels in the spines. Consistent with this, NMDA receptor activation in neurons impairs the ability of GABAB receptors to activate K+ channels. Thus, our data support that NMDA receptor activity endocytoses postsynaptic GABAB receptors through CaMKII-mediated phosphorylation of S867. This provides a means to spare NMDA receptors at individual glutamatergic synapses from reciprocal inhibition through GABAB receptors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
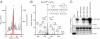

Similar articles
-
Ca2+/Calmodulin-Dependent Protein Kinase II (CaMKII) β-Dependent Phosphorylation of GABAB1 Triggers Lysosomal Degradation of GABAB Receptors via Mind Bomb-2 (MIB2)-Mediated Lys-63-Linked Ubiquitination.Mol Neurobiol. 2019 Feb;56(2):1293-1309. doi: 10.1007/s12035-018-1142-5. Epub 2018 Jun 7. Mol Neurobiol. 2019. PMID: 29881949 Free PMC article.
-
Prolonged activation of NMDA receptors promotes dephosphorylation and alters postendocytic sorting of GABAB receptors.Proc Natl Acad Sci U S A. 2010 Aug 3;107(31):13918-23. doi: 10.1073/pnas.1000853107. Epub 2010 Jul 19. Proc Natl Acad Sci U S A. 2010. PMID: 20643948 Free PMC article.
-
ERK1/2-Dependent Phosphorylation of GABAB1(S867/T872), Controlled by CaMKIIβ, Is Required for GABAB Receptor Degradation under Physiological and Pathological Conditions.Int J Mol Sci. 2023 Aug 30;24(17):13436. doi: 10.3390/ijms241713436. Int J Mol Sci. 2023. PMID: 37686242 Free PMC article.
-
The role of CaMKII autophosphorylation for NMDA receptor-dependent synaptic potentiation.Neuropharmacology. 2021 Aug 1;193:108616. doi: 10.1016/j.neuropharm.2021.108616. Epub 2021 May 26. Neuropharmacology. 2021. PMID: 34051268 Review.
-
GABAB receptors: modulation of thalamocortical dynamics and synaptic plasticity.Neuroscience. 2021 Feb 21;456:131-142. doi: 10.1016/j.neuroscience.2020.03.011. Epub 2020 Mar 17. Neuroscience. 2021. PMID: 32194227 Review.
Cited by
-
Complex GABAB receptor complexes: how to generate multiple functionally distinct units from a single receptor.Front Pharmacol. 2014 Feb 11;5:12. doi: 10.3389/fphar.2014.00012. eCollection 2014. Front Pharmacol. 2014. PMID: 24575041 Free PMC article. Review.
-
Racemization in Post-Translational Modifications Relevance to Protein Aging, Aggregation and Neurodegeneration: Tip of the Iceberg.Symmetry (Basel). 2021 Mar;13(3):455. doi: 10.3390/sym13030455. Epub 2021 Mar 11. Symmetry (Basel). 2021. PMID: 34350031 Free PMC article.
-
Pharmacological mechanism and clinical application of ciprofol.Front Pharmacol. 2025 Mar 25;16:1572112. doi: 10.3389/fphar.2025.1572112. eCollection 2025. Front Pharmacol. 2025. PMID: 40201700 Free PMC article. Review.
-
Methamphetamine-evoked depression of GABA(B) receptor signaling in GABA neurons of the VTA.Neuron. 2012 Mar 8;73(5):978-89. doi: 10.1016/j.neuron.2011.12.031. Neuron. 2012. PMID: 22405207 Free PMC article.
-
Impact of perinatal dioxin exposure on infant growth: a cross-sectional and longitudinal studies in dioxin-contaminated areas in Vietnam.PLoS One. 2012;7(7):e40273. doi: 10.1371/journal.pone.0040273. Epub 2012 Jul 16. PLoS One. 2012. PMID: 22815734 Free PMC article.
References
-
- Bettler B, Kaupmann K, Mosbacher J, Gassmann M. Molecular structure and physiological functions of GABAB receptors. Physiol Rev. 2004;84:835–867. - PubMed
-
- Couve A, Moss SJ, Pangalos MN. GABAB receptors: A new paradigm in G protein signaling. Mol Cell Neurosci. 2000;16:296–312. - PubMed
-
- Ulrich D, Bettler B. GABAB receptors: Synaptic functions and mechanisms of diversity. Curr Opin Neurobiol. 2007;17:298–303. - PubMed
-
- Kornau HC. GABAB receptors and synaptic modulation. Cell Tissue Res. 2006;326:517–533. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

