Distinct roles for two synaptotagmin isoforms in synchronous and asynchronous transmitter release at zebrafish neuromuscular junction
- PMID: 20643933
- PMCID: PMC2922265
- DOI: 10.1073/pnas.1008598107
Distinct roles for two synaptotagmin isoforms in synchronous and asynchronous transmitter release at zebrafish neuromuscular junction
Abstract
An obligatory role for the calcium sensor synaptotagmins in stimulus-coupled release of neurotransmitter is well established, but a role for synaptotagmin isoform involvement in asynchronous release remains conjecture. We show, at the zebrafish neuromuscular synapse, that two separate synaptotagmins underlie these processes. Specifically, knockdown of synaptotagmin 2 (syt2) reduces synchronous release, whereas knockdown of synaptotagmin 7 (syt7) reduces the asynchronous component of release. The zebrafish neuromuscular junction is unique in having a very small quantal content and a high release probability under conditions of either low-frequency stimulation or high-frequency augmentation. Through these features, we further determined that during the height of shared synchronous and asynchronous transmission these two modes compete for the same release sites.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

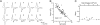



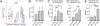
References
-
- Feng TP. The changes in the endplate potential during and after prolonged stimulation. Chin J Physiol. 1941;13:29.
-
- Eccles JC, Katz B, Kuffler SW. Nature of the endplate potentials in curarized muscle. J Neurophysiol. 1941;4:6.
-
- Lu T, Trussell LO. Inhibitory transmission mediated by asynchronous transmitter release. Neuron. 2000;26:683–694. - PubMed
-
- Hagler DJ, Jr, Goda Y. Properties of synchronous and asynchronous release during pulse train depression in cultured hippocampal neurons. J Neurophysiol. 2001;85:2324–2334. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

