Alterations in the immuno-skeletal interface drive bone destruction in HIV-1 transgenic rats
- PMID: 20643942
- PMCID: PMC2922243
- DOI: 10.1073/pnas.1003020107
Alterations in the immuno-skeletal interface drive bone destruction in HIV-1 transgenic rats
Abstract
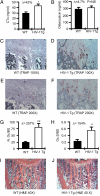
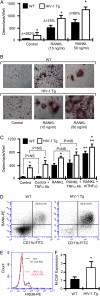
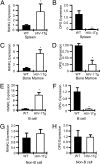
Osteoporosis and bone fractures are increasingly recognized complications of HIV-1 infection. Although antiretroviral therapy itself has complex effects on bone turnover, it is now evident that the majority of HIV-infected individuals already exhibit reduced bone mineral density before therapy. The mechanisms responsible are likely multifactorial and have been difficult to delineate in humans. The HIV-1 transgenic rat recapitulates many key features of human AIDS. We now demonstrate that, like their human counterparts, HIV-1 transgenic rats undergo severe osteoclastic bone resorption, a consequence of an imbalance in the ratio of receptor activator of NF-kappaB ligand, the key osteoclastogenic cytokine, to that of its physiological decoy receptor osteoprotegerin. This imbalance stemmed from a switch in production of osteoprotegerin to that of receptor activator of NF-kappaB ligand by B cells, and was further compounded by a significantly elevated number of osteoclast precursors. With the advancing age of individuals living with HIV/AIDS, low bone mineral density associated with HIV infection is likely to collide with the pathophysiology of skeletal aging, leading to increased fracture risk. Understanding the mechanisms driving bone loss in HIV-infected individuals will be critical to developing effective therapeutic strategies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Deeks SG. Immune dysfunction, inflammation, and accelerated aging in patients on antiretroviral therapy. Top HIV Med. 2009;17:118–123. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 AR046031/AR/NIAMS NIH HHS/United States
- MZ AR053898/AR/NIAMS NIH HHS/United States
- AR056090/AR/NIAMS NIH HHS/United States
- R01 AR059364/AR/NIAMS NIH HHS/United States
- UL1 TR000454/TR/NCATS NIH HHS/United States
- R01 AR056090/AR/NIAMS NIH HHS/United States
- AR059364/AR/NIAMS NIH HHS/United States
- I01 BX000105/BX/BLRD VA/United States
- UL1 RR025008/RR/NCRR NIH HHS/United States
- U01 AI103408/AI/NIAID NIH HHS/United States
- P30-AR46031/AR/NIAMS NIH HHS/United States
- R38 AI140299/AI/NIAID NIH HHS/United States
- AR053607/AR/NIAMS NIH HHS/United States
- U54 AG062334/AG/NIA NIH HHS/United States
- R21 AR053607/AR/NIAMS NIH HHS/United States
- R01 AR053898/AR/NIAMS NIH HHS/United States
- K23 A1073119/PHS HHS/United States
- K23 AI073119/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

