Osteoinductive ceramics as a synthetic alternative to autologous bone grafting
- PMID: 20643969
- PMCID: PMC2922269
- DOI: 10.1073/pnas.1003600107
Osteoinductive ceramics as a synthetic alternative to autologous bone grafting
Abstract
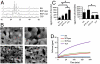
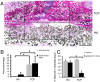
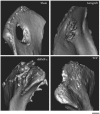
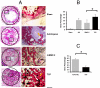
Biomaterials can be endowed with biologically instructive properties by changing basic parameters such as elasticity and surface texture. However, translation from in vitro proof of concept to clinical application is largely missing. Porous calcium phosphate ceramics are used to treat small bone defects but in general do not induce stem cell differentiation, which is essential for regenerating large bone defects. Here, we prepared calcium phosphate ceramics with varying physicochemical and structural characteristics. Microporosity correlated to their propensity to stimulate osteogenic differentiation of stem cells in vitro and bone induction in vivo. Implantation in a large bone defect in sheep unequivocally demonstrated that osteoinductive ceramics are equally efficient in bone repair as autologous bone grafts. Our results provide proof of concept for the clinical application of "smart" biomaterials.
Conflict of interest statement
Conflict of interest statement: H.Y., J.D.d.B., and C.A.v.B. are share-holders of Progentix Orthobiology.
Figures


References
-
- Chen CS, Mrksich M, Huang S, Whitesides GM, Ingber DE. Geometric control of cell life and death. Science. 1997;276(5317):1425–1428. - PubMed
-
- Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126(4):677–689. - PubMed
-
- McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev Cell. 2004;6(4):483–495. - PubMed
-
- Folkman J, Moscona A. Role of cell shape in growth control. Nature. 1978;273(5661):345–349. - PubMed
-
- Place ES, Evans ND, Stevens MM. Complexity in biomaterials for tissue engineering. Nat Mater. 2009;8(6):457–470. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

