Characterization of cytokine-induced myeloid-derived suppressor cells from normal human peripheral blood mononuclear cells
- PMID: 20644162
- PMCID: PMC2923483
- DOI: 10.4049/jimmunol.1000901
Characterization of cytokine-induced myeloid-derived suppressor cells from normal human peripheral blood mononuclear cells
Erratum in
- J Immunol. 2010 Nov 1;185(9):5668
Abstract
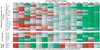
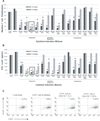
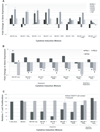
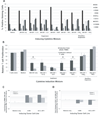
Tumor immune tolerance can derive from the recruitment of suppressor cell populations, including myeloid-derived suppressor cells (MDSCs). In cancer patients, increased MDSCs correlate with more aggressive disease and a poor prognosis. Expression of 15 immune factors (TGFbeta, IL-1beta, IL-4, IL-6, IL-10, GM-CSF, M-CSF, IDO, fms-related tyrosine kinase 3 ligand, c-kit ligand, inducible NO synthase, arginase-1, TNF-alpha, cyclo-oxygenase 2, vascular endothelial growth factor [VEGF]) by MDSC-inducing human solid tumor cell lines was evaluated by RT-PCR. Based upon these data, cytokine mixtures were then tested for their ability to generate suppressive CD33(+) cells from healthy donor PBMCs in vitro by measuring their ability to inhibit the proliferation of, and IFN-gamma production by, fresh autologous human T cells after CD3/CD28 stimulation. Induced MDSCs were characterized with respect to their morphology, surface phenotype, and gene expression profile. MDSC-inducing cancer cell lines demonstrated multiple pathways for MDSC generation, including overexpression of IL-6, IL-1beta, cyclo-oxygenase 2, M-CSF, and IDO. CD33(+) cells with potent suppressive capacity were best generated in vitro by GM-CSF and IL-6, and secondarily by GM-CSF + IL-1beta, PGE(2), TNF-alpha, or VEGF. Characterization studies of cytokine-induced suppressive cells revealed CD33(+)CD11b(+)CD66b(+)HLA-DR(low)IL-13R alpha2(int) large mononuclear cells with abundant basophilic cytoplasm. Expression of inducible NO synthase, TGFbeta, NADPH oxidase, VEGF, and/or arginase-1 was also upregulated, and Transwell studies showed suppression of autologous T cells to be contact dependent. Suppressive CD33(+) cells generated from PBMCs by GM-CSF and IL-6 were consistent with human MDSCs. This study suggests that these cytokines are potential therapeutic targets for the inhibition of MDSC induction in cancer patients.
Figures

References
-
- Stewart TJ, Abrams SI. How tumours escape mass destruction. Oncogene. 2008;27:5894–5903. - PubMed
-
- Serafini P, Borrello I, Bronte V. Myeloid suppressor cells in cancer: recruitment, phenotype, properties, and mechanisms of immune suppression. Semin Cancer Biol. 2006;16:53–65. - PubMed
-
- Ugel S, Delpozzo F, Desantis G, Papalini F, Simonato F, Sonda N, Zilio S, Bronte V. Therapeutic targeting of myeloid-derived suppressor cells. Curr Opin Pharmacol. 2009;9:470–481. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

