Deep brain stimulation results in local glutamate and adenosine release: investigation into the role of astrocytes
- PMID: 20644423
- PMCID: PMC2919357
- DOI: 10.1227/01.NEU.0000371988.73620.4C
Deep brain stimulation results in local glutamate and adenosine release: investigation into the role of astrocytes
Abstract
Background: Several neurological disorders are treated with deep brain stimulation; however, the mechanism underlying its ability to abolish oscillatory phenomena associated with diseases as diverse as Parkinson's disease and epilepsy remain largely unknown.
Objective: To investigate the role of specific neurotransmitters in deep brain stimulation and determine the role of non-neuronal cells in its mechanism of action.
Methods: We used the ferret thalamic slice preparation in vitro, which exhibits spontaneous spindle oscillations, to determine the effect of high-frequency stimulation on neurotransmitter release. We then performed experiments using an in vitro astrocyte culture to investigate the role of glial transmitter release in high-frequency stimulation-mediated abolishment of spindle oscillations.
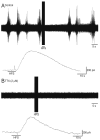
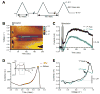
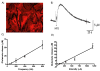
Results: In this series of experiments, we demonstrated that glutamate and adenosine release in ferret slices was able to abolish spontaneous spindle oscillations. The glutamate release was still evoked in the presence of the Na channel blocker tetrodotoxin, but was eliminated with the vesicular H-ATPase inhibitor bafilomycin and the calcium chelator 2-bis(2-aminophenoxy)-ethane-N,N,N',N'-tetraacetic acid tetrakis acetoxymethyl ester. Furthermore, electrical stimulation of purified primary astrocytic cultures was able to evoke intracellular calcium transients and glutamate release, and bath application of 2-bis (2-aminophenoxy)-ethane-N,N,N',N'-tetraacetic acid tetrakis acetoxymethyl ester inhibited glutamate release in this setting.
Conclusion: Vesicular astrocytic neurotransmitter release may be an important mechanism by which deep brain stimulation is able to achieve clinical benefits.
Figures



Similar articles
-
High frequency stimulation abolishes thalamic network oscillations: an electrophysiological and computational analysis.J Neural Eng. 2011 Aug;8(4):046001. doi: 10.1088/1741-2560/8/4/046001. Epub 2011 May 27. J Neural Eng. 2011. PMID: 21623007 Free PMC article.
-
Abolition of spindle oscillations and 3-Hz absence seizurelike activity in the thalamus by using high-frequency stimulation: potential mechanism of action.J Neurosurg. 2005 Sep;103(3):538-45. doi: 10.3171/jns.2005.103.3.0538. J Neurosurg. 2005. PMID: 16235687
-
Hippocampal astrocytes in situ respond to glutamate released from synaptic terminals.J Neurosci. 1996 Aug 15;16(16):5073-81. doi: 10.1523/JNEUROSCI.16-16-05073.1996. J Neurosci. 1996. PMID: 8756437 Free PMC article.
-
Neuroprotective use-dependent blockers of Na+ and Ca2+ channels controlling presynaptic release of glutamate.Ann N Y Acad Sci. 1995 Sep 15;765:210-29. doi: 10.1111/j.1749-6632.1995.tb16578.x. Ann N Y Acad Sci. 1995. PMID: 7486608 Review.
-
Do astrocytes really exocytose neurotransmitters?Nat Rev Neurosci. 2010 Apr;11(4):227-38. doi: 10.1038/nrn2803. Nat Rev Neurosci. 2010. PMID: 20300101 Review.
Cited by
-
Selective Antagonism of A1 Adenosinergic Receptors Strengthens the Neuromodulation of the Sensorimotor Network During Epidural Spinal Stimulation.Front Syst Neurosci. 2020 Jul 14;14:44. doi: 10.3389/fnsys.2020.00044. eCollection 2020. Front Syst Neurosci. 2020. PMID: 32760254 Free PMC article.
-
The Role of Astroglia in the Antidepressant Action of Deep Brain Stimulation.Front Cell Neurosci. 2016 Jan 12;9:509. doi: 10.3389/fncel.2015.00509. eCollection 2015. Front Cell Neurosci. 2016. PMID: 26793061 Free PMC article. No abstract available.
-
Global and multi-focal changes in cerebral blood flow during subthalamic nucleus stimulation in Parkinson's disease.J Cereb Blood Flow Metab. 2018 Apr;38(4):697-705. doi: 10.1177/0271678X17705042. Epub 2017 Apr 19. J Cereb Blood Flow Metab. 2018. PMID: 28421851 Free PMC article.
-
The Role of Astrocyte Dysfunction in Parkinson's Disease Pathogenesis.Trends Neurosci. 2017 Jun;40(6):358-370. doi: 10.1016/j.tins.2017.04.001. Epub 2017 May 17. Trends Neurosci. 2017. PMID: 28527591 Free PMC article. Review.
-
Modulation of Glia-Mediated Processes by Spinal Cord Stimulation in Animal Models of Neuropathic Pain.Front Pain Res (Lausanne). 2021 Jul 14;2:702906. doi: 10.3389/fpain.2021.702906. eCollection 2021. Front Pain Res (Lausanne). 2021. PMID: 35295479 Free PMC article. Review.
References
-
- Krack P, Batir A, Van Blercom N, et al. Five-year follow-up of bilateral stimulation of the subthalamic nucleus in advanced Parkinson's disease. N Engl J Med. 2003 Nov 13;349(20):1925–1934. - PubMed
-
- Bronte-Stewart H. Surgical therapy for dystonia. Curr Neurol Neurosci Rep. 2003 Jul;3(4):296–305. - PubMed
-
- Bereznai B, Steude U, Seelos K, Botzel K. Chronic high-frequency globus pallidus internus stimulation in different types of dystonia: a clinical, video, and MRI report of six patients presenting with segmental, cervical, and generalized dystonia. Mov Disord. 2002 Jan;17(1):138–144. - PubMed
-
- Kupsch A, Benecke R, Muller J, et al. Pallidal deep-brain stimulation in primary generalized or segmental dystonia. N Engl J Med. 2006 Nov 9;355(19):1978–1990. - PubMed
-
- Koller WC, Pahwa PR, Lyons KE, Wilkinson SB. Deep brain stimulation of the Vim nucleus of the thalamus for the treatment of tremor. Neurology. 2000;55(12 Suppl 6):S29–33. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

