Withagulatin A inhibits hepatic stellate cell viability and procollagen I production through Akt and Smad signaling pathways
- PMID: 20644552
- PMCID: PMC4007817
- DOI: 10.1038/aps.2010.72
Withagulatin A inhibits hepatic stellate cell viability and procollagen I production through Akt and Smad signaling pathways
Abstract
Aim: To investigate the effects of the natural product Withagulatin A on hepatic stellate cell (HSC) viability and type I procollagen production. The potential mechanism underlying the pharmacological actions was also explored.
Methods: The effect of Withagulatin A on cell viability was evaluated in HSC and LX-2 cells using a sulforhodamine B (SRB) assay. Cell cycle distribution was analyzed using flow cytometry. Type I procollagen gene expression was determined using real-time PCR. Regulation of signaling molecules by Withagulatin A was detected using Western blotting.
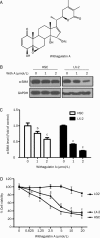
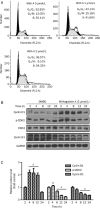
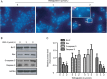
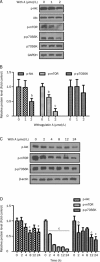
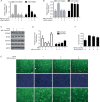
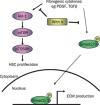
Results: Primary rat HSCs and the human hepatic stellate cell line LX-2 treated with Withagulatin A (0.625-20 micromol/L) underwent a dose-dependent decrease in cell viability, which was associated with S phase arrest and the induction of cell apoptosis. In addition, the natural product decreased phosphorylation of the Akt/mTOR/p70S6K pathway that controls cell proliferation and survival. Furthermore, Withagulatin A (1, 2 mumol/L) inhibited transforming growth factor-beta (TGF-beta) stimulated type I procollagen gene expression, which was attributable to the suppression of TGF-beta stimulated Smad2 and Smad3 phosphorylation.
Conclusion: Our results demonstrated that Withagulatin A potently inhibited HSC viability and type I procollagen production, thereby implying that this natural product has potential use in the development of anti-fibrogenic reagents for the treatment of hepatic fibrosis.
Figures
Similar articles
-
Antifibrotic effects of luteolin on hepatic stellate cells and liver fibrosis by targeting AKT/mTOR/p70S6K and TGFβ/Smad signalling pathways.Liver Int. 2015 Apr;35(4):1222-33. doi: 10.1111/liv.12638. Epub 2014 Aug 5. Liver Int. 2015. PMID: 25040634
-
Lipopolysaccharides induce Smad2 phosphorylation through PI3K/Akt and MAPK cascades in HSC-T6 hepatic stellate cells.Life Sci. 2017 Sep 1;184:37-46. doi: 10.1016/j.lfs.2017.07.004. Epub 2017 Jul 6. Life Sci. 2017. PMID: 28689803
-
Methyl ferulic acid attenuates liver fibrosis and hepatic stellate cell activation through the TGF-β1/Smad and NOX4/ROS pathways.Chem Biol Interact. 2019 Feb 1;299:131-139. doi: 10.1016/j.cbi.2018.12.006. Epub 2018 Dec 11. Chem Biol Interact. 2019. PMID: 30543783
-
Liuweiwuling tablets attenuate BDL-induced hepatic fibrosis via modulation of TGF-β/Smad and NF-κB signaling pathways.J Ethnopharmacol. 2018 Jan 10;210:232-241. doi: 10.1016/j.jep.2017.08.029. Epub 2017 Aug 31. J Ethnopharmacol. 2018. PMID: 28864168
-
Leukamenin F suppresses liver fibrogenesis by inhibiting both hepatic stellate cell proliferation and extracellular matrix production.Acta Pharmacol Sin. 2010 Jul;31(7):839-48. doi: 10.1038/aps.2010.64. Epub 2010 Jun 21. Acta Pharmacol Sin. 2010. PMID: 20562900 Free PMC article.
Cited by
-
Plumbagin protects liver against fulminant hepatic failure and chronic liver fibrosis via inhibiting inflammation and collagen production.Oncotarget. 2016 Dec 13;7(50):82864-82875. doi: 10.18632/oncotarget.12655. Oncotarget. 2016. PMID: 27756878 Free PMC article.
-
Inhibition Effect of Physalis angulata Leaf Extract on Viability, Collagen Type I, and Tissue Inhibitor of Metalloproteinase 1 (TIMP-1) but Not Plasminogen Activator Inhibitor-1 (PAI-1) of Keloid Fibroblast Culture.Clin Cosmet Investig Dermatol. 2023 Aug 30;16:2365-2373. doi: 10.2147/CCID.S425036. eCollection 2023. Clin Cosmet Investig Dermatol. 2023. PMID: 37667736 Free PMC article.
-
Characterization of the conversion between CD133+ and CD133- cells in colon cancer SW620 cell line.Cancer Biol Ther. 2012 Dec;13(14):1396-406. doi: 10.4161/cbt.22000. Epub 2012 Sep 6. Cancer Biol Ther. 2012. PMID: 22954703 Free PMC article.
References
-
- Albanis E, Friedman SL. Hepatic fibrosis. Pathogenesis and principles of therapy. Clin Liver Dis. 2001;5:315–34. v–vi. - PubMed
-
- Friedman SL. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J Biol Chem. 2000;275:2247–50. - PubMed
-
- Geerts A, Lazou JM, De Bleser P, Wisse E. Tissue distribution, quantitation and proliferation kinetics of fat-storing cells in carbon tetrachloride-injured rat liver. Hepatology. 1991;13:1193–202. - PubMed
-
- Mak KM, Leo MA, Lieber CS. Alcoholic liver injury in baboons: transformation of lipocytes to transitional cells. Gastroenterology. 1984;87:188–200. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

