Time to update and quantitative changes in the results of cochrane pregnancy and childbirth reviews
- PMID: 20644625
- PMCID: PMC2903478
- DOI: 10.1371/journal.pone.0011553
Time to update and quantitative changes in the results of cochrane pregnancy and childbirth reviews
Abstract
Background: The recommended interval between updates for systematic reviews included in The Cochrane Library is 2 years. However, it is unclear whether this interval is always appropriate. Whereas excessive updating wastes time and resources, insufficient updating allows out-of-date or incomplete evidence to guide clinical decision-making. We set out to determine, for Cochrane pregnancy and childbirth reviews, the frequency of updates, factors associated with updating, and whether updating frequency was appropriate.
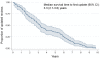
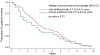
Methodology/principal findings: Cochrane pregnancy and childbirth reviews published in Issue 3, 2007 of the Cochrane Database of Systematic Reviews were retrieved, and data were collected from their original and updated versions. Quantitative changes were determined for one of the primary outcomes (mortality, or the outcome of greatest clinical significance). Potential factors associated with time to update were assessed using the Cox proportional hazard model. Among the 101 reviews in our final sample, the median time before the first update was 3.3 years (95% CI 2.7-3.8). Only 32.7% had been updated within the recommended interval of 2 years. In 75.3% (76/101), a median of 3 new trials with a median of 576 additional participants were included in the updated versions. There were quantitative changes in 71% of the reviews that included new trials (54/76): the median change in effect size was 18.2%, and the median change in 95% CI width was 30.8%. Statistical significance changed in 18.5% (10/54) of these reviews, but conclusions were revised in only 3.7% (2/54). A shorter time to update was associated with the same original review team at updating.
Conclusions/significance: Most reviews were updated less frequently than recommended by Cochrane policy, but few updates had revised conclusions. Prescribed time to update should be reconsidered to support improved decision-making while making efficient use of limited resources.
Conflict of interest statement
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources