Efficacy and safety of inhaled carbon monoxide during pulmonary inflammation in mice
- PMID: 20644637
- PMCID: PMC2903490
- DOI: 10.1371/journal.pone.0011565
Efficacy and safety of inhaled carbon monoxide during pulmonary inflammation in mice
Abstract
Background: Pulmonary inflammation is a major contributor to morbidity in a variety of respiratory disorders, but treatment options are limited. Here we investigate the efficacy, safety and mechanism of action of low dose inhaled carbon monoxide (CO) using a mouse model of lipopolysaccharide (LPS)-induced pulmonary inflammation.
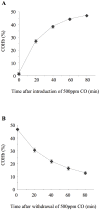
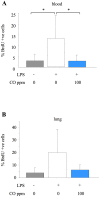
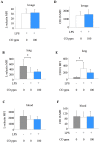
Methodology: Mice were exposed to 0-500 ppm inhaled CO for periods of up to 24 hours prior to and following intratracheal instillation of 10 ng LPS. Animals were sacrificed and assessed for intraalveolar neutrophil influx and cytokine levels, flow cytometric determination of neutrophil number and activation in blood, lung and lavage fluid samples, or neutrophil mobilisation from bone marrow.
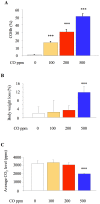
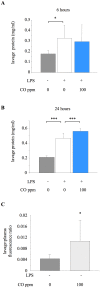
Principal findings: When administered for 24 hours both before and after LPS, inhaled CO of 100 ppm or more reduced intraalveolar neutrophil infiltration by 40-50%, although doses above 100 ppm were associated with either high carboxyhemoglobin, weight loss or reduced physical activity. This anti-inflammatory effect of CO did not require pre-exposure before induction of injury. 100 ppm CO exposure attenuated neutrophil sequestration within the pulmonary vasculature as well as LPS-induced neutrophilia at 6 hours after LPS, likely due to abrogation of neutrophil mobilisation from bone marrow. In contrast to such apparently beneficial effects, 100 ppm inhaled CO induced an increase in pulmonary barrier permeability as determined by lavage fluid protein content and translocation of labelled albumin from blood to the alveolar space.
Conclusions: Overall, these data confirm some protective role for inhaled CO during pulmonary inflammation, although this required a dose that produced carboxyhemoglobin values close to potentially toxic levels for humans, and increased lung permeability.
Conflict of interest statement
Figures




References
-
- Ryter SW, Alam J, Choi AM. Heme oxygenase-1/carbon monoxide: from basic science to therapeutic applications. Physiol Rev. 2006;86:583–650. - PubMed
-
- Chapman JT, Otterbein LE, Elias JA, Choi AM. Carbon monoxide attenuates aeroallergen-induced inflammation in mice. Am J Physiol Lung Cell Mol Physiol. 2001;281:L209–216. - PubMed
-
- Dolinay T, Szilasi M, Liu M, Choi AM. Inhaled carbon monoxide confers antiinflammatory effects against ventilator-induced lung injury. Am J Respir Crit Care Med. 2004;170:613–620. - PubMed
-
- Kohmoto J, Nakao A, Stolz DB, Kaizu T, Tsung A, et al. Carbon monoxide protects rat lung transplants from ischemia-reperfusion injury via a mechanism involving p38 MAPK pathway. Am J Transplant. 2007;7:2279–2290. - PubMed

