Activation of hypoxia inducible factor 1 is a general phenomenon in infections with human pathogens
- PMID: 20644645
- PMCID: PMC2904385
- DOI: 10.1371/journal.pone.0011576
Activation of hypoxia inducible factor 1 is a general phenomenon in infections with human pathogens
Abstract
Background: Hypoxia inducible factor (HIF)-1 is the key transcriptional factor involved in the adaptation process of cells and organisms to hypoxia. Recent findings suggest that HIF-1 plays also a crucial role in inflammatory and infectious diseases.
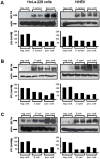
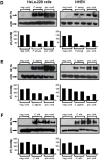
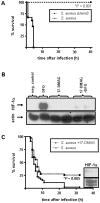
Methodology/principal findings: Using patient skin biopsies, cell culture and murine infection models, HIF-1 activation was determined by immunohistochemistry, immunoblotting and reporter gene assays and was linked to cellular oxygen consumption. The course of a S. aureus peritonitis was determined upon pharmacological HIF-1 inhibition. Activation of HIF-1 was detectable (i) in all ex vivo in biopsies of patients suffering from skin infections, (ii) in vitro using cell culture infection models and (iii) in vivo using murine intravenous and peritoneal S. aureus infection models. HIF-1 activation by human pathogens was induced by oxygen-dependent mechanisms. Small colony variants (SCVs) of S. aureus known to cause chronic infections did not result in cellular hypoxia nor in HIF-1 activation. Pharmaceutical inhibition of HIF-1 activation resulted in increased survival rates of mice suffering from a S. aureus peritonitis.
Conclusions/significance: Activation of HIF-1 is a general phenomenon in infections with human pathogenic bacteria, viruses, fungi and protozoa. HIF-1-regulated pathways might be an attractive target to modulate the course of life-threatening infections.
Conflict of interest statement
Figures






References
-
- Shimoda LA, Manalo DJ, Sham JS, Semenza GL, Sylvester JT. Partial HIF-1alpha deficiency impairs pulmonary arterial myocyte electrophysiological responses to hypoxia. Am J Physiol Lung Cell Mol Physiol. 2001;281:202–208. - PubMed
-
- Pugh CW, Ratcliffe PJ. Regulation of angiogenesis by hypoxia: role of the HIF system. Nat Med. 2003;9:677–684. - PubMed
-
- Ivan M, Kondo K, Yang H, Kim W, Valiando J, et al. HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science. 2001;292:464–468. - PubMed
-
- Mazure NM, Brahimi-Horn MC, Berta MA, Benizri E, Bilton RL, et al. HIF-1: master and commander of the hypoxic world. A pharmacological approach to its regulation by siRNAs. Biochem Pharmacol. 2004;68:971–980. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

