Modeling of dielectrophoretic transport of myoglobin molecules in microchannels
- PMID: 20644674
- PMCID: PMC2905271
- DOI: 10.1063/1.3339773
Modeling of dielectrophoretic transport of myoglobin molecules in microchannels
Abstract
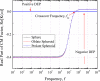
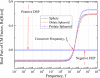
Myoglobin is one of the premature identifying cardiac markers, whose concentration increases from 90 pgml or less to over 250 ngml in the blood serum of human beings after minor heart attack. Separation, detection, and quantification of myoglobin play a vital role in revealing the cardiac arrest in advance, which is the challenging part of ongoing research. In the present work, one of the electrokinetic approaches, i.e., dielectrophoresis (DEP), is chosen to separate the myoglobin. A mathematical model is developed for simulating dielectrophoretic behavior of a myoglobin molecule in a microchannel to provide a theoretical basis for the above application. This model is based on the introduction of a dielectrophoretic force and a dielectric myoglobin model. A dielectric myoglobin model is developed by approximating the shape of the myoglobin molecule as sphere, oblate, and prolate spheroids. A generalized theoretical expression for the dielectrophoretic force acting on respective shapes of the molecule is derived. The microchannel considered for analysis has an array of parallel rectangular electrodes at the bottom surface. The potential and electric field distributions are calculated using Green's theorem method and finite element method. These results also compared to the Fourier series method, closed form solutions by Morgan et al. [J. Phys. D: Appl. Phys. 34, 1553 (2001)] and Chang et al. [J. Phys. D: Appl. Phys. 36, 3073 (2003)]. It is observed that both Green's theorem based analytical solution and finite element based numerical solution for proposed model are closely matched for electric field and square electric field gradients. The crossover frequency is obtained as 40 MHz for given properties of myoglobin and for all approximated shapes of myoglobin molecule. The effect of conductivity of medium and myoglobin on the crossover frequency is also demonstrated. Further, the effect of hydration layer on the crossover frequency of myoglobin molecules is also presented. Both positive and negative DEP effects on myoglobin molecules are obtained by switching the frequency of applied electric field. The effect of different shapes of myoglobin on DEP force is studied and no significant effect on DEP force is observed. Finally, repulsion of myoglobin molecules from the electrode plane at 1 KHz frequency and 10 V applied voltage is observed. These results provide the ability of applying DEP force for manipulating nanosized biomolecules such as myoglobin.
Figures











References
-
- Evan J., Novel data analysis technique aids heart attack diagnosis, February 2006, see also URL Lab Informatics, separationsNOW.com.
-
- Darain F., Yager P., Gan K. L., and Tjin S. C., Biosens. Bioelectron. 24, 1744 (2009). - PubMed
-
- Perkoff G. T., Hill R. L., Brown D. M., and Tyler F. H., J. Biol. Chem. 237, 2820 (1962). - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
