The role of spatially controlled cell proliferation in limb bud morphogenesis
- PMID: 20644711
- PMCID: PMC2903592
- DOI: 10.1371/journal.pbio.1000420
The role of spatially controlled cell proliferation in limb bud morphogenesis
Abstract
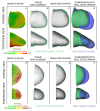
Although the vertebrate limb bud has been studied for decades as a model system for spatial pattern formation and cell specification, the cellular basis of its distally oriented elongation has been a relatively neglected topic by comparison. The conventional view is that a gradient of isotropic proliferation exists along the limb, with high proliferation rates at the distal tip and lower rates towards the body, and that this gradient is the driving force behind outgrowth. Here we test this hypothesis by combining quantitative empirical data sets with computer modelling to assess the potential role of spatially controlled proliferation rates in the process of directional limb bud outgrowth. In particular, we generate two new empirical data sets for the mouse hind limb--a numerical description of shape change and a quantitative 3D map of cell cycle times--and combine these with a new 3D finite element model of tissue growth. By developing a parameter optimization approach (which explores spatial patterns of tissue growth) our computer simulations reveal that the observed distribution of proliferation rates plays no significant role in controlling the distally extending limb shape, and suggests that directional cell activities are likely to be the driving force behind limb bud outgrowth. This theoretical prediction prompted us to search for evidence of directional cell orientations in the limb bud mesenchyme, and we thus discovered a striking highly branched and extended cell shape composed of dynamically extending and retracting filopodia, a distally oriented bias in Golgi position, and also a bias in the orientation of cell division. We therefore provide both theoretical and empirical evidence that limb bud elongation is achieved by directional cell activities, rather than a PD gradient of proliferation rates.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures







References
-
- Wolpert L. The French Flag problem: a contribution to the discussion on pattern development and regulation; In: Waddington C. H, editor. Edinburgh: Edinburgh University Press; 1968.
-
- Wolpert L. Positional information and the spatial pattern of cellular differentiation. J Theor Biol. 1969;25:1–47. - PubMed
-
- Gasseling M. T, Saunders J. W., Jr Effects of the apical ectodermal ridge on growth of the versene-stripped chick limb bud. Dev Biol. 1961;3:1–25. - PubMed
-
- Bell E, Saunders J. W, Jr, Zwilling E. Limb development in the absence of ectodermal ridge. Nature. 1959;184(Suppl 22):1736–1737. - PubMed
-
- Saunders J. W., Jr Is the progress zone model a victim of progress? Cell. 2002;110:541–543. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

