Convenient and reproducible in vivo gene transfer to mouse parotid glands
- PMID: 20646229
- PMCID: PMC3010376
- DOI: 10.1111/j.1601-0825.2010.01707.x
Convenient and reproducible in vivo gene transfer to mouse parotid glands
Abstract
Objectives: Published studies of gene transfer to mouse salivary glands have not employed the parotid glands. Parotid glands are the likely target tissue for most clinical applications of salivary gene transfer. The purpose of the present study was to develop a convenient and reproducible method of retroductal gene transfer to mouse parotid glands.
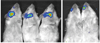
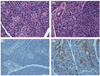
Methods: The volume for vector delivery was assessed by infusion of Toluidine Blue into Stensen's ducts of Balb/c mice after direct intraoral cannulation. Recombinant, serotype 5 adenoviral vectors, encoding either firefly luciferase or human erythropoietin (hEpo), were constructed and then administered to parotid glands (10(7) vector particles/gland). Transgene expression in vivo was measured by enzyme activity (luciferase) or an enzyme-linked immunosorbent assay (hEpo). Vector biodistribution was measured by real-time quantitative (Q) PCR.
Results: The chosen volume for mouse parotid vector delivery was 20μL. Little vector was detected outside of the targeted glands, with both QPCR and luciferase assays. Transgene expression was readily detected in glands (luciferase, hEpo), and serum and saliva (hEpo). Most secreted hEpo was detected in saliva.
Conclusion: These studies show that mouse parotid glands can be conveniently and reproducibly targeted for gene transfer, and should be useful for pre-clinical studies with many murine disease models.
© 2010 John Wiley & Sons A/S.
Figures



Similar articles
-
Sorting of transgenic secretory proteins in miniature pig parotid glands following adenoviral-mediated gene transfer.J Gene Med. 2007 Sep;9(9):779-87. doi: 10.1002/jgm.1081. J Gene Med. 2007. PMID: 17654745
-
Sorting of transgenic secretory proteins in rhesus macaque parotid glands after adenovirus-mediated gene transfer.Hum Gene Ther. 2008 Dec;19(12):1401-5. doi: 10.1089/hum.2008.034. Hum Gene Ther. 2008. PMID: 18764738 Free PMC article.
-
Long-term transduction of miniature pig parotid glands using serotype 2 adeno-associated viral vectors.J Gene Med. 2009 Jun;11(6):506-14. doi: 10.1002/jgm.1319. J Gene Med. 2009. PMID: 19326368 Free PMC article.
-
Transfer of the AQP1 cDNA for the correction of radiation-induced salivary hypofunction.Biochim Biophys Acta. 2006 Aug;1758(8):1071-7. doi: 10.1016/j.bbamem.2005.11.006. Epub 2005 Dec 5. Biochim Biophys Acta. 2006. PMID: 16368071 Review.
-
Salivary epithelial cells: an unassuming target site for gene therapeutics.Int J Biochem Cell Biol. 2010 Jun;42(6):773-7. doi: 10.1016/j.biocel.2010.02.012. Epub 2010 Feb 26. Int J Biochem Cell Biol. 2010. PMID: 20219693 Free PMC article. Review.
Cited by
-
A novel hybrid adenoretroviral vector with more extensive E3 deletion extends transgene expression in submandibular glands.Hum Gene Ther Methods. 2012 Jun;23(3):169-81. doi: 10.1089/hgtb.2011.175. Epub 2012 Jul 20. Hum Gene Ther Methods. 2012. PMID: 22817829 Free PMC article.
-
Protective MCMV immunity by vaccination of the salivary gland via Wharton's duct: replication-deficient recombinant adenovirus expressing individual MCMV genes elicits protection similar to that of MCMV.FASEB J. 2014 Apr;28(4):1698-710. doi: 10.1096/fj.13-244178. Epub 2014 Jan 3. FASEB J. 2014. PMID: 24391133 Free PMC article.
-
Paracrine effects of bone marrow soup restore organ function, regeneration, and repair in salivary glands damaged by irradiation.PLoS One. 2013 Apr 24;8(4):e61632. doi: 10.1371/journal.pone.0061632. Print 2013. PLoS One. 2013. PMID: 23637870 Free PMC article.
-
Retroductal Nanoparticle Injection to the Murine Submandibular Gland.J Vis Exp. 2018 May 3;(135):57521. doi: 10.3791/57521. J Vis Exp. 2018. PMID: 29781991 Free PMC article.
-
Plasmid DNA is internalized from the apical plasma membrane of the salivary gland epithelium in live animals.Histochem Cell Biol. 2012 Aug;138(2):201-13. doi: 10.1007/s00418-012-0959-7. Epub 2012 Apr 29. Histochem Cell Biol. 2012. PMID: 22544351 Free PMC article.
References
-
- Adesanya MR, Redman RS, Baum BJ, O’Connell BC. Immediate inflammatory responses to adenovirus-mediated gene transfer in rat salivary glands. Hum Gene Ther. 1996;7:1085–1093. - PubMed
-
- Barka T, van der Noen H. Retrovirus-mediated gene transfer into salivary glands in vivo. Hum Gene Ther. 1996;7:613–618. - PubMed
-
- Baum BJ, Wellner RB, Zheng C. Gene transfer to salivary glands. Int Rev Cytol. 2002;213:93–146. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

