Pathologic nodal evaluation improves prognostic accuracy in Merkel cell carcinoma: analysis of 5823 cases as the basis of the first consensus staging system
- PMID: 20646783
- PMCID: PMC2956767
- DOI: 10.1016/j.jaad.2010.02.056
Pathologic nodal evaluation improves prognostic accuracy in Merkel cell carcinoma: analysis of 5823 cases as the basis of the first consensus staging system
Abstract
Background: The management of Merkel cell carcinoma (MCC) has been complicated by a lack of detailed prognostic data and by the presence of conflicting staging systems.
Objective: We sought to determine the prognostic significance of tumor size, clinical versus pathologic nodal evaluation, and extent of disease at presentation and thereby derive the first consensus staging/prognostic system for MCC.
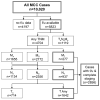
Methods: A total of 5823 prospectively enrolled MCC cases from the National Cancer Data Base had follow-up data (median 64 months) and were used for prognostic analyses.
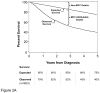
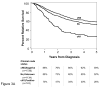
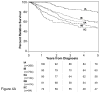
Results: At 5 years, overall survival was 40% and relative survival (compared with age- and sex-matched population data) was 54%. Among all MCC cases, 66% presented with local, 27% with nodal, and 7% with distant metastatic disease. For cases presenting with local disease only, smaller tumor size was associated with better survival (stage I, ≤2 cm, 66% relative survival at 5 years; stage II, >2 cm, 51%; P < .0001). Patients with clinically local-only disease and pathologically proven negative nodes had better outcome (76% at 5 years) than those who only underwent clinical nodal evaluation (59%, P < .0001).
Limitations: The National Cancer Data Base does not capture disease-specific survival. Overall survival for patients with MCC was therefore used to calculate relative survival based on matched population data.
Conclusion: Although the majority (68%) of patients with MCC in this nationwide cohort did not undergo pathologic nodal evaluation, this procedure may be indicated in many cases as it improves prognostic accuracy and has important treatment implications for those found to have microscopic nodal involvement.
Copyright © 2010 American Academy of Dermatology, Inc. Published by Mosby, Inc. All rights reserved.
Conflict of interest statement
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST: The author(s) indicated no potential conflicts of interest.
Figures






References
-
- Hodgson NC. Merkel cell carcinoma: changing incidence trends. J Surg Oncol. 2005;89(1):1–4. - PubMed
-
- Lemos B, Nghiem P. Merkel cell carcinoma: more deaths but still no pathway to blame. J Invest Dermatol. 2007;127(9):2100–3. - PubMed
-
- Engels EA, Frisch M, Goedert JJ, Biggar RJ, Miller RW. Merkel cell carcinoma and HIV infection. Lancet. 2002;359(9305):497–8. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

