Alcohol impairs interferon signaling and enhances full cycle hepatitis C virus JFH-1 infection of human hepatocytes
- PMID: 20646875
- PMCID: PMC2967585
- DOI: 10.1016/j.drugalcdep.2010.05.008
Alcohol impairs interferon signaling and enhances full cycle hepatitis C virus JFH-1 infection of human hepatocytes
Abstract
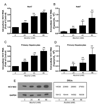
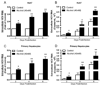
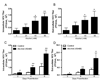
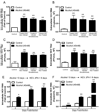
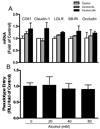
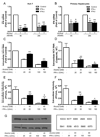
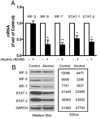
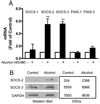
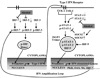
Alcohol drinking and hepatitis C virus (HCV) infection frequently coexist in patients with chronic liver disease. There is limited information, however, about the impact of alcohol on host cell innate immunity and full cycle replication of HCV. This study investigated whether alcohol impairs the intracellular innate immunity in human hepatocytes, promoting HCV infection and replication. Alcohol treatment of human hepatocytes before, during and after viral infection significantly enhanced full cycle HCV replication. Alcohol suppressed intracellular expression of type I interferons (IFN-α/β) in human hepatocytes. Investigation of the mechanisms responsible for the alcohol action revealed that alcohol inhibited the expression of the IFN regulatory factors (IRF-5 and IRF-7), and signal transducer and activator of transcription (STAT-1 and STAT-2), the key positive regulators in type I IFN signaling pathway. In addition, alcohol induced the expression of suppressors of cytokine signaling (SOCS-2 and SOCS-3), the key negative regulators of IFN-α/β expression. These in vitro findings suggest that alcohol, through modulating the expression of key regulators in IFN signaling pathway, inhibits type I IFN-based intracellular innate immunity in hepatocytes, which may contribute to the chronicity of HCV infection and the poor efficacy of IFN-α-based therapy.
Copyright © 2010 Elsevier Ireland Ltd. All rights reserved.
Figures
Similar articles
-
Retinoic acid inducible gene-I (RIG-I) signaling of hepatic stellate cells inhibits hepatitis C virus replication in hepatocytes.Innate Immun. 2013;19(2):193-202. doi: 10.1177/1753425912460414. Epub 2012 Oct 11. Innate Immun. 2013. PMID: 23060457 Free PMC article.
-
Effect of ethanol on innate antiviral pathways and HCV replication in human liver cells.Virol J. 2005 Dec 2;2:89. doi: 10.1186/1743-422X-2-89. Virol J. 2005. PMID: 16324217 Free PMC article.
-
Hepatitis C virus replication in mouse cells is restricted by IFN-dependent and -independent mechanisms.Gastroenterology. 2013 Dec;145(6):1414-23.e1. doi: 10.1053/j.gastro.2013.08.037. Epub 2013 Aug 21. Gastroenterology. 2013. PMID: 23973921
-
Impact of alcohol on hepatitis C virus replication and interferon signaling.World J Gastroenterol. 2010 Mar 21;16(11):1337-43. doi: 10.3748/wjg.v16.i11.1337. World J Gastroenterol. 2010. PMID: 20238400 Free PMC article. Review.
-
Understanding the molecular mechanism(s) of hepatitis C virus (HCV) induced interferon resistance.Infect Genet Evol. 2013 Oct;19:113-9. doi: 10.1016/j.meegid.2013.06.025. Epub 2013 Jul 5. Infect Genet Evol. 2013. PMID: 23831932 Review.
Cited by
-
Hepatitis C, innate immunity and alcohol: friends or foes?Biomolecules. 2015 Feb 5;5(1):76-94. doi: 10.3390/biom5010076. Biomolecules. 2015. PMID: 25664450 Free PMC article. Review.
-
Inhibition of TLR8- and TLR4-induced Type I IFN induction by alcohol is different from its effects on inflammatory cytokine production in monocytes.BMC Immunol. 2011 Sep 30;12:55. doi: 10.1186/1471-2172-12-55. BMC Immunol. 2011. PMID: 21962237 Free PMC article.
-
Alcohol-induced autophagy via upregulation of PIASy promotes HCV replication in human hepatoma cells.Cell Death Dis. 2018 Sep 5;9(9):898. doi: 10.1038/s41419-018-0845-x. Cell Death Dis. 2018. PMID: 30185779 Free PMC article.
-
Involvement of autophagy in alcoholic liver injury and hepatitis C pathogenesis.World J Gastroenterol. 2011 May 28;17(20):2507-14. doi: 10.3748/wjg.v17.i20.2507. World J Gastroenterol. 2011. PMID: 21633655 Free PMC article. Review.
-
Alcohol enhances HIV infection of cord blood monocyte-derived macrophages.Curr HIV Res. 2014;12(4):301-8. doi: 10.2174/1570162x12666140721124923. Curr HIV Res. 2014. PMID: 25053361 Free PMC article.
References
-
- Ahluwalia B, Wesley B, Adeyiga O, Smith DM, Da-Silva A, Rajguru S. Alcohol modulates cytokine secretion and synthesis in human fetus: an in vivo and in vitro study. Alcohol. 2000;21:207–213. - PubMed
-
- Alter MJ. Prevention of spread of hepatitis C. Hepatology. 2002;36:S93–S98. - PubMed
-
- Barnes BJ, Moore PA, Pitha PM. Virus-specific activation of a novel interferon regulatory factor, IRF-5, results in the induction of distinct interferon alpha genes. J Biol Chem. 2001;276:23382–23390. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

