Clinical and immunologic predictors of influenza illness among vaccinated older adults
- PMID: 20646987
- PMCID: PMC2948951
- DOI: 10.1016/j.vaccine.2010.07.036
Clinical and immunologic predictors of influenza illness among vaccinated older adults
Abstract
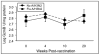
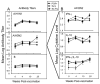
The diagnosis of influenza is often missed in older adults and illness presentation may be modified by prior vaccination. We evaluated the symptoms and immunologic markers predicting laboratory-confirmed influenza (LCI) among vaccinated older adults. In subjects with influenza-like illness (ILI), fever distinguished subjects with laboratory-confirmed influenza (LCI) from those with other ILI (39% vs. 12.5%, p=0.009). In LCI subjects who did not seroconvert to influenza infection, pre-infection levels of the cytolytic mediator, granzyme B, correlated with fever (r=1.000; p=0.01) and the IFN-gamma:IL-10 ratio (r=0.999; p=0.03), and increased following influenza infection in LCI vs. ILI subjects (p=0.03). The cell-mediated immune response to influenza distinguishes A/H3N2 LCI from other ILI in older adults, and suggests a link between cell-mediated immunity and influenza illness severity in vaccinated older adults.
(c) 2010 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Conflict of interest: Dr. McEhaney has received funding for this study under an investigated-initiated research contract with GSK. She has also received funding for the following (alphabetical order): CSL (honoraria), Dynavax (honoraria), GSK (consultancy, honoraria and research funding), Merck (consultancy, honoraria, participation in clinical research study and trial), Novartis (honoraria), Novavax (consultancy), Sanofi Pasteur (consultancy and honoraria), and Solvay (consultancy and honoraria).
Figures

References
-
- Thompson WW, Shay DK, Weintraub E, Brammer L, Cox N, Anderson LJ, et al. Mortality associated with influenza and respiratory syncytial virus in the United States. Jama. 2003;289(2):179–86. - PubMed
-
- Thompson WW, Shay DK, Weintraub E, Brammer L, Bridges CB, Cox NJ, et al. Influenza-associated hospitalizations in the United States. Jama. 2004;292(11):1333–40. - PubMed
-
- Glezen WP. Serious morbidity and mortality associated with influenza epidemics. Epidemiol Rev. 1982;4:25–44. - PubMed
-
- Carrat F, Tachet A, Rouzioux C, Housset B, Valleron AJ. Evaluation of clinical case definitions of influenza: detailed investigation of patients during the 1995–1996 epidemic in France. Clin Infect Dis. 1999;28(2):283–90. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

