Development of the retina and optic pathway
- PMID: 20647017
- PMCID: PMC2974959
- DOI: 10.1016/j.visres.2010.07.010
Development of the retina and optic pathway
Abstract
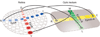
Our understanding of the development of the retina and visual pathways has seen enormous advances during the past 25years. New imaging technologies, coupled with advances in molecular biology, have permitted a fuller appreciation of the histotypical events associated with proliferation, fate determination, migration, differentiation, pathway navigation, target innervation, synaptogenesis and cell death, and in many instances, in understanding the genetic, molecular, cellular and activity-dependent mechanisms underlying those developmental changes. The present review considers those advances associated with the lineal relationships between retinal nerve cells, the production of retinal nerve cell diversity, the migration, patterning and differentiation of different types of retinal nerve cells, the determinants of the decussation pattern at the optic chiasm, the formation of the retinotopic map, and the establishment of ocular domains within the thalamus.
Copyright © 2010 Elsevier Ltd. All rights reserved.
Figures
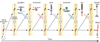





References
-
- Adler R. A model of retinal cell differentiation in the chick embryo. Prog. Ret. Eye Res. 2000;19:529–557. - PubMed
-
- Agathocleous M, Harris WA. Cell determination. In: Sernagor E, Eglen S, Harris B, Wong R, editors. Retinal Development. Cambridge: Cambridge University Press; 2006. pp. 75–98.
-
- Agathocleous M, Harris WA. From progenitors to differentiated cells in the vertebrate retina. Ann. Rev. Cell Develop. Biol. 2009;25:45–69. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

